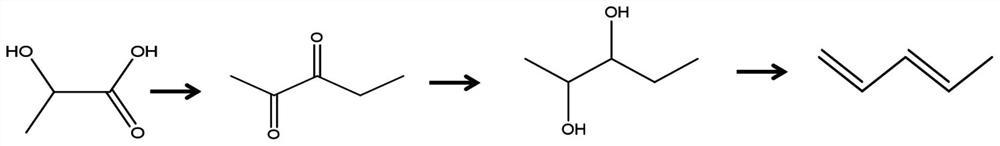
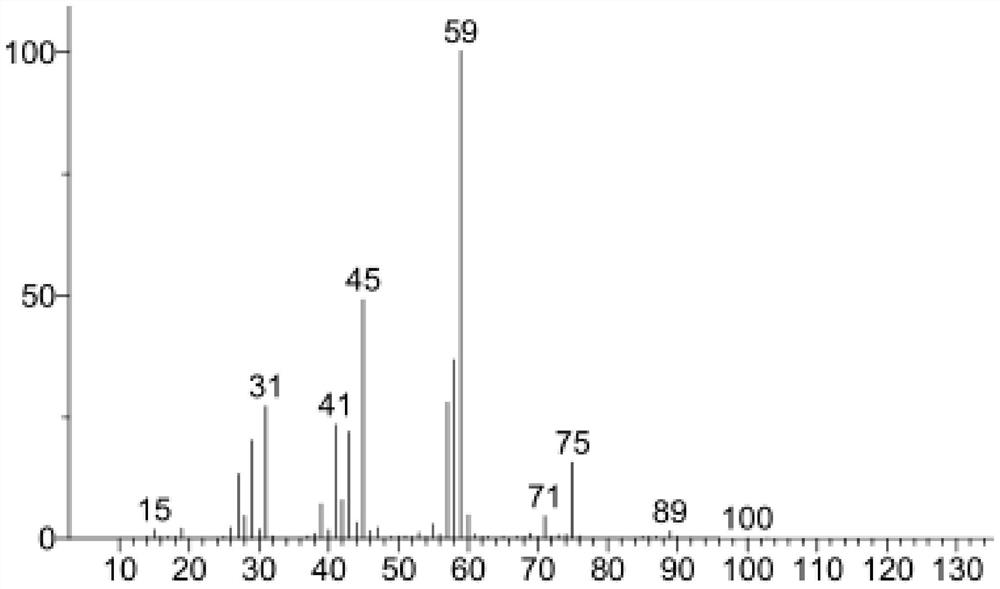
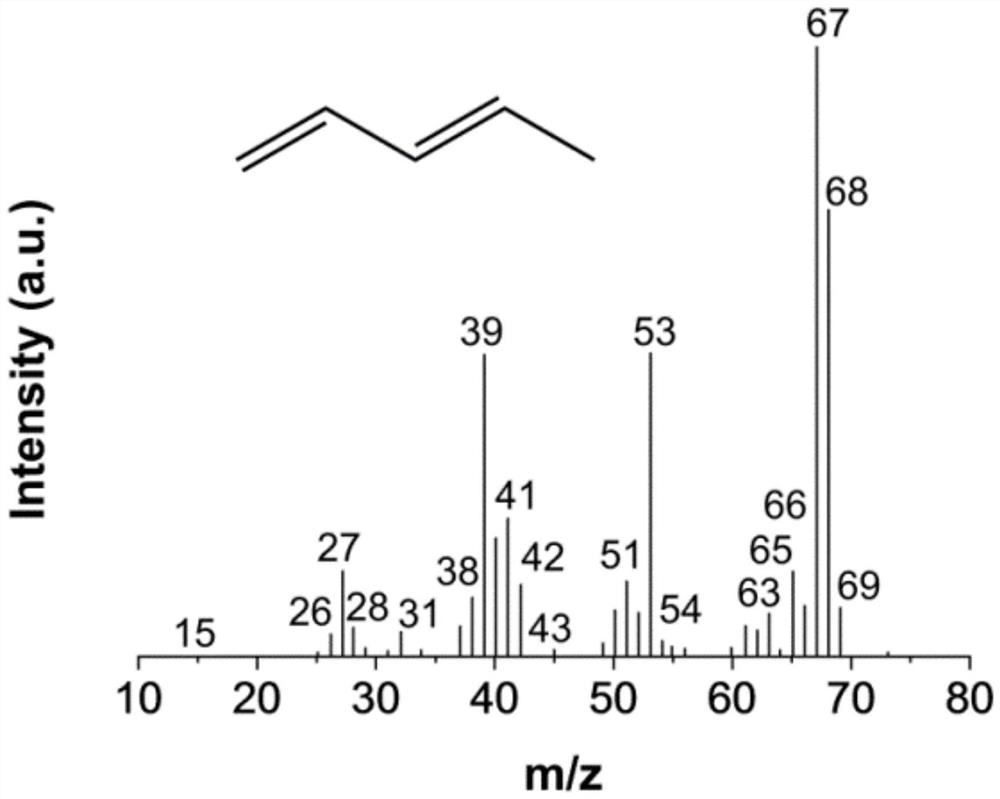
Synthesis method of pentanediol and synthesis method for preparing biomass-based linear pentadiene based on lactic acid conversion
A synthetic method, the technology of pentanediol, which is applied in the direction of condensation preparation of carbonyl compounds, carbon-based compound preparation, hydroxyl compound preparation, etc., can solve the problems of low industrial value, complicated process and low yield of linear pentadiene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] The invention provides a kind of synthetic method of pentanediol, comprising the following steps:
[0087] 1) The mixed solution of pentanedione, hydrogenation catalyst and organic solvent is subjected to hydrogenation reaction in an atmosphere containing hydrogen to obtain pentanediol.
[0088] In principle, the present invention has no special limitation on the amount of the hydrogenation catalyst, and those skilled in the art can select and adjust according to actual application needs, product requirements and quality requirements. The present invention is to better ensure the green synthesis of products and improve the reaction rate For efficiency, the concentration of the hydrogenation catalyst in the mixed liquid is preferably 1-30 mg / ml, more preferably 6-25 mg / ml, and more preferably 11-20 mg / ml.
[0089] In principle, the present invention has no special restrictions on the amount of pentanedione in the mixed solution, and those skilled in the art can select an...
Embodiment 1
[0205] (1) Lactic acid condensation reaction to prepare pentadione:
[0206] Weigh 2.0g of sodium bicarbonate to prepare 5ml of solution, add 5g of MgO under stirring condition, let stand for 12h, and dry at 120°C for 10h. Before use, MgO was calcined at 550°C for 4 hours to obtain a condensation catalyst. Weigh 1.5g of the catalyst and load it into a fixed-bed reactor; before the reaction, the catalyst is placed at 550°C, and the treatment time is preferably 3h; during the reaction, the feed rate of the raw material is 0.06ml / min, the nitrogen flow rate is 60ml / min, and the reaction temperature is 380°C ℃. The obtained pentanedione yield was 73%, and the raw material conversion rate was 89%.
[0207] (2) Pentanedione Hydrogenation to Pentylene Glycol Reaction:
[0208] Will Al 2 o 3 After the carrier was calcined at 500°C for 5h, weigh 3.0g into a beaker, add 3ml of chloroplatinic acid solution, stir for 10min, let stand overnight, dry in an oven at 120°C for 8h, then ca...
Embodiment 2
[0212] (1) Lactic acid condensation reaction to prepare pentadione:
[0213]Weigh 1.8g potassium bicarbonate and configure it into 5ml solution, add 5g Al 2 o 3 , let stand for 12h, and dry at 120°C for 10h. Al before use 2 o 3 Calcined at 600°C for 5h to obtain a condensation catalyst. Weigh 2.0 g of the catalyst and load it into a fixed-bed reactor; before the reaction, the catalyst is placed at 500 ° C, and the treatment time is preferably 4 hours; during the reaction, the feed rate of the raw material is 0.08 ml / min, the nitrogen flow rate is 60 ml / min, and the reaction temperature is 300 °C ℃. The obtained pentanedione yield is 62%, and the raw material conversion rate is 93%.
[0214] (2) Pentanedione Hydrogenation to Pentylene Glycol Reaction:
[0215] ZrO 2 After the carrier is calcined at 500°C for 5 hours, weigh 3.0g into a beaker, add 3ml of cerium nitrate solution, control the loading capacity of cerium nitrate to 18%, let stand overnight, dry at 120°C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com