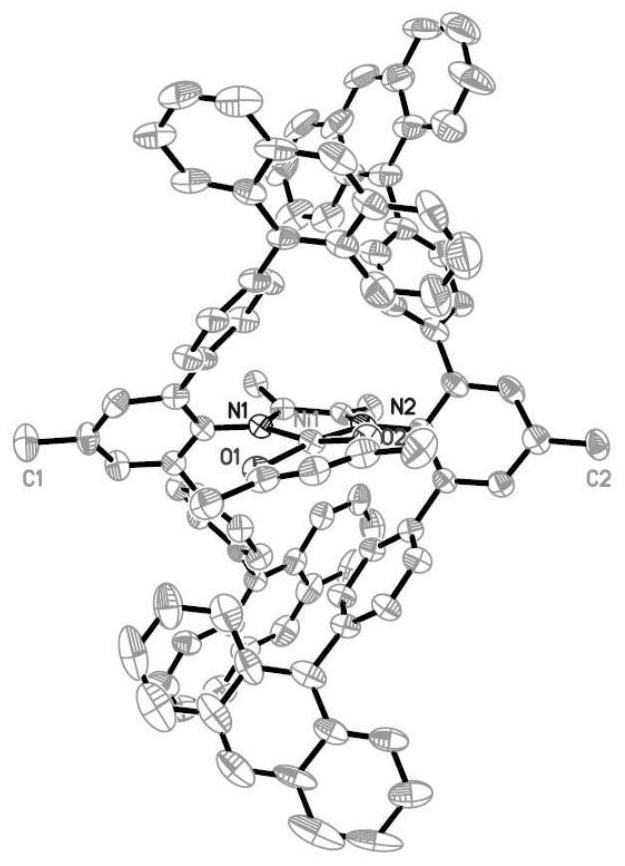
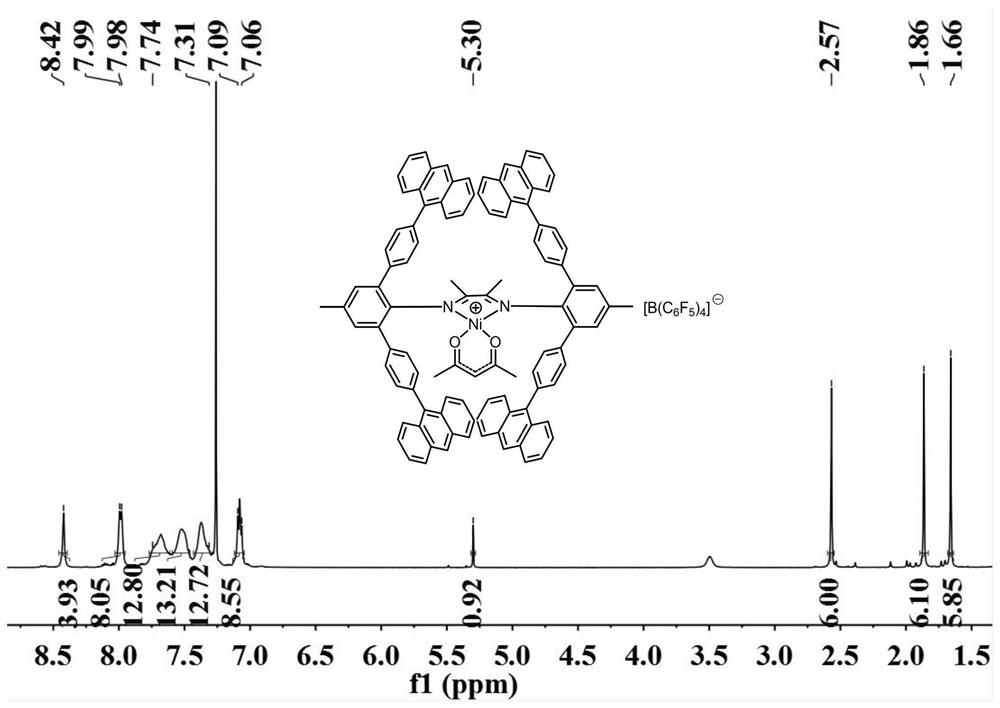
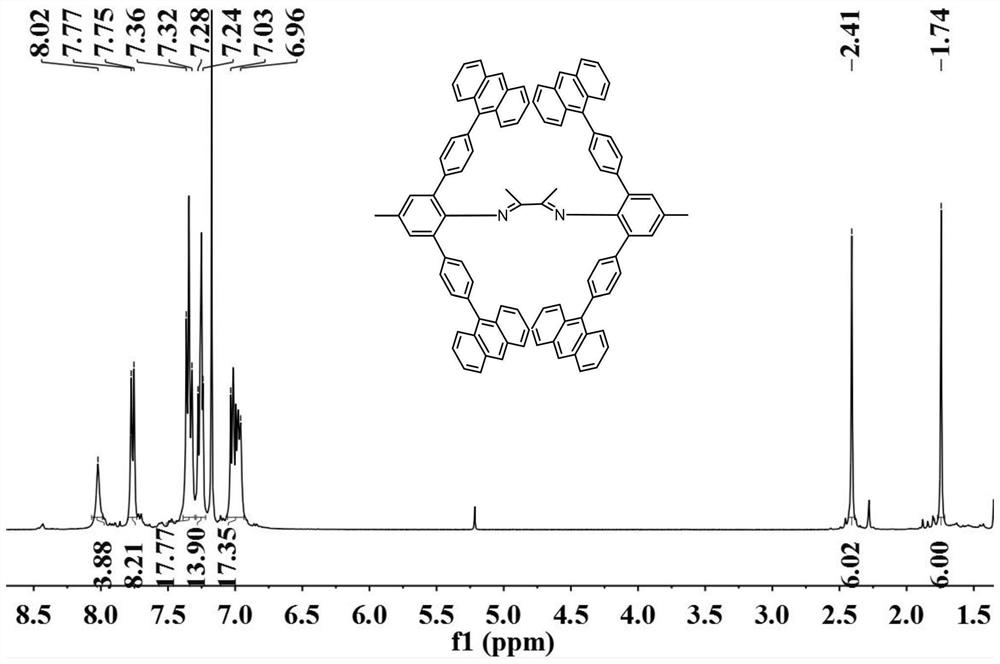
Aromatic amine based on rigid terphenyl structure, alpha-diimine ligand, nickel catalyst and preparation method and application thereof
A technology of diimine ligand and terphenyl, which is applied in the preparation of imino compounds, chemical instruments and methods, preparation of amino compounds from amines, etc., can solve the problem of hindering the insertion of polar monomers and unfavorable for the synthesis of functionalized polyolefin materials , can not significantly improve the polymerization effect and other problems, to achieve the effect of excellent catalytic performance, excellent thermal stability, molecular weight and thermal stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Step 1, the preparation of 4-methyl-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) aniline
[0074]
[0075] Under an inert atmosphere (nitrogen), 2,6-dibromo-4-methylaniline (0.50g, 1.9mmol), bis(pinacol)diborane (1.44g, 5.7mmol), potassium acetate (0.55g , 5.6mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (0.028g) were dissolved in dimethyl sulfoxide (20mL), then stirred at 80°C for 24 hours or more. After the reaction was completed, the black-red liquid mixture was poured into ice water (50 mL) and the precipitate was filtered. The precipitate was redissolved in dichloromethane, the organic layer was washed with water, separated and dried over anhydrous sodium sulfate. After the solid was filtered, the organic phase was concentrated by rotary evaporation in vacuo. By flash column chromatography (silica gel; PE / CH 2 Cl 2 = 3:1) to purify the resulting residue and then use CH 2 Cl 2 / CH 3 Recrystallization from OH afforded 0.40 g (60%...
Embodiment 2
[0090] Step 1-Step 5 are the same as in Example 1, except for the variables in Tables 1-4, other conditions are unchanged. In the table, the substitute for 4-methyl-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)aniline of Example 1 is designated A , the substitute of 4-anthracenyl bromobenzene is recorded as B, the substitute of 4-methyl-2,6-bis[(4-anthracenyl)phenyl]aniline is recorded as C,2,3-butanedione The substitute is denoted as D, the substitute of bis-[2,6-bis(4-anthracenyl)phenyl-4-methylphenyl)butanedione-1,2-diimine is denoted as E, [( MeN^N)NiBr 2 and [(MeN^N)Ni(acac)][B(C 6 f 5 ) 4 ] The substitute is denoted as F.
[0091] Table 1 Reactants of Step 2 Synthesis C (reaction temperature: 90°C, reaction time: 24h)
[0092]
[0093]
[0094]
[0095] Table 2 Reactants of step 3 synthesis E (reaction temperature: 130°C, reaction time: 48h)
[0096]
[0097]
[0098]
[0099]
[0100] Table 3 Step 4 Synthesis of F reactants (reaction time:...
Embodiment 3
[0110] A 350 mL glass pressure reactor connected to a high pressure gas line was first vacuum dried at 90 °C for at least 1 h. Then adjust the reactor to 30°C, add 98 mL of toluene and 500 μmol of MMAO into the reactor under an inert atmosphere, and then dissolve 1 μmol of Ni catalyst in 2 mL of dichloromethane (or chloroform) and inject it into the polymerization system through a syringe middle. Under rapid stirring (over 750 rpm), ethylene was introduced and kept at 8 atm. After 10 minutes, the pressure reactor was emptied, and a large amount of ethanol (or methanol) solution with a concentration of 5 wt% hydrochloric acid was added to quench the polymerization reaction, filtered, and dried in a vacuum oven to constant weight.
[0111] Wherein, the structural general formula of nickel catalyst is shown in formula (Ⅲ), R 2 =CH 3 , R 3 = R 4 =H,R 5 = Anthracene, R 1 , R 10 , R 11 See Table 5.
[0112] Different nickel catalysts under table 5 high pressure (change sub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com