Aggregation-induced-emission polypeptide-micelle-type diagnostic reagent and application thereof in near-infrared-region biological imaging
A technology of aggregation-induced luminescence and diagnostic reagents, which can be applied to preparations, peptides, and drug delivery for in vivo experiments. It can solve the problems of lack of tumor targeting, reduced biological safety, and complex synthesis process, and achieve biodegradability. Diversified properties and functions, high yield, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
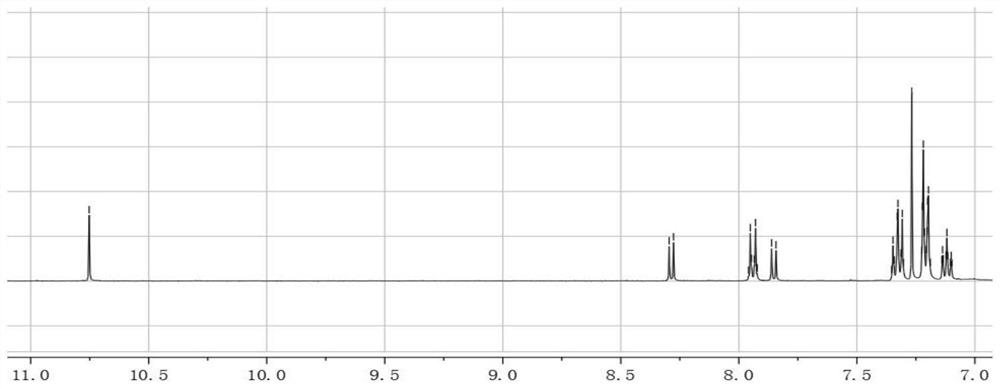
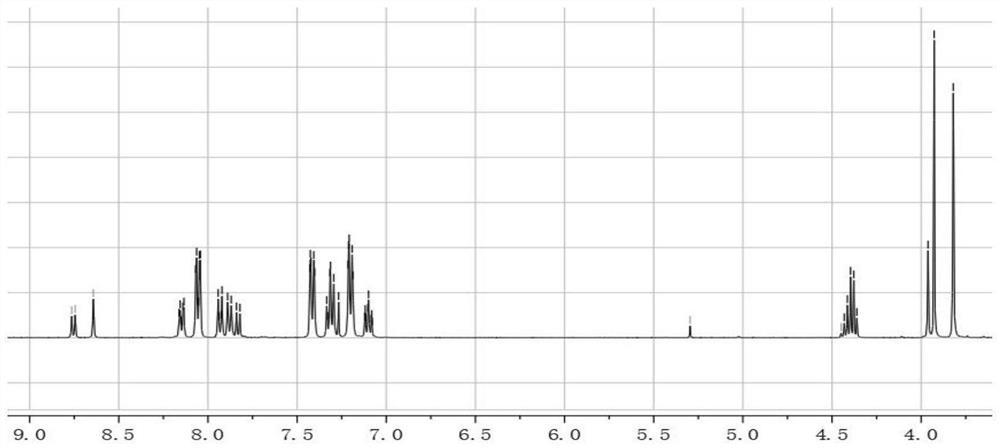
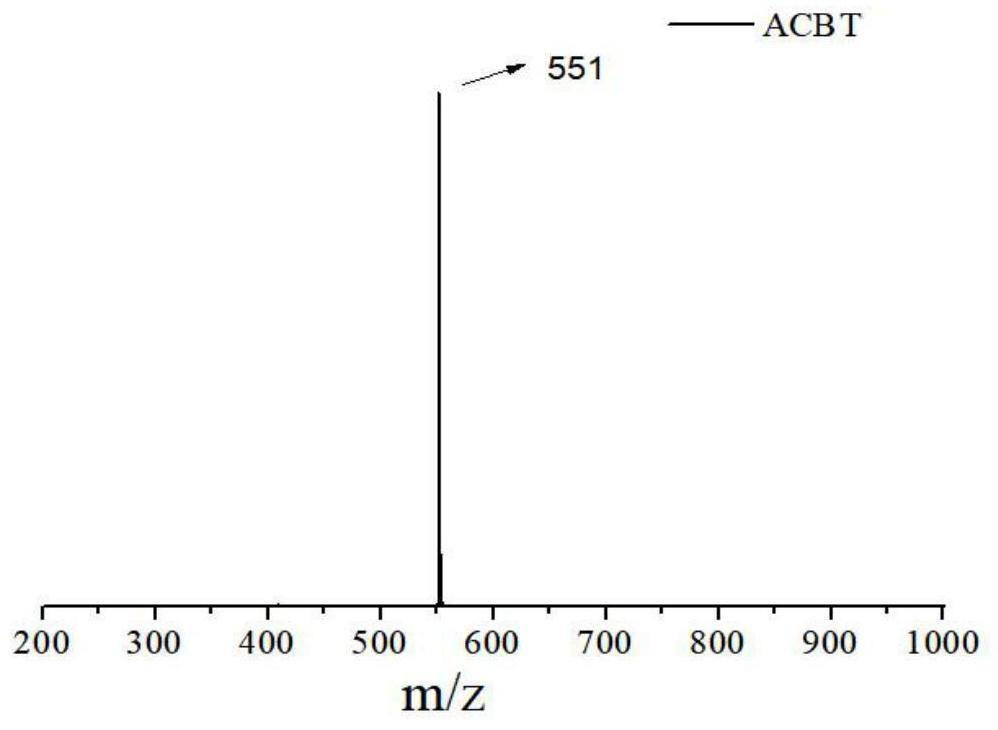
[0057] The synthesis of embodiment one, ACBT
[0058] Synthesize ACBT according to the following synthetic route:
[0059]
[0060] The synthesis steps of ACBT are:
[0061] 7-bromobenzo[c][1,2,5]thiadiazole-4-carbaldehyde (abbreviated as ABB) (1mmol), 4-triphenylamine borate (2mmol), anhydrous sodium carbonate (1.06g, 10mmol ), tetrakis(triphenylphosphine)palladium (50mg, 0.04mmol) was put into a three-necked flask, and nitrogen gas was fed to remove the oxygen in the flask. Then, 20 mL of toluene and 3 mL of water were injected into the flask under a nitrogen atmosphere, and the flask was placed in an oil bath at 110° C. for reflux reaction for 24 h. After the reaction, 40 mL of dichloromethane and 4.0 g of silica gel powder were added, and spun into powder using a rotary evaporator. Compound 1 (abbreviated as ABT) was purified by silica gel column chromatography, using a mixed solvent of dichloromethane and petroleum ether with a volume ratio of 3:1 as the eluent, and...
Embodiment 2
[0064] Example 2. Synthesis of aggregation-induced luminescent polypeptide micellar diagnostic reagent AGPR
[0065] 1. The functional chimeric peptide GGFLG-PEG was synthesized by the standard Fmoc solid-phase peptide synthesis method 8 -R 8GD: Weigh 2-chloro-trityl chloride resin (0.8g, 0.97mmol / g) into the peptide solid-phase synthesis column, add 20 mL N,N-dimethylformamide (DMF) to soak the resin for 30 minutes, Make it fully swell and remove DMF by suction filtration. Prepare to insert the first amino acid and enter the amino acid condensation reaction. Then, a DMF solution in which Fmoc-Asp(OtBu)-OH (3 times the degree of substitution of the resin) and DIEA (6 equivalents of the degree of substitution of the resin) was dissolved was added to the polypeptide solid-phase synthesis column, and reacted for 2 hours. After the reaction, the filtrate was sucked away and washed 4 times with DMF. The peptide resin after condensation of the first amino acid protected by FMOC ...
Embodiment 3
[0069] Example 3. Self-assembly performance and morphology characterization of aggregation-induced luminescent polypeptide micellar diagnostic reagent AGPR
[0070] Using pyrene as a hydrophobic fluorescent probe, the critical micelle concentration (CMC) value of AGPR was measured by fluorescence spectrophotometer to evaluate the self-assembly performance and stability of AGPR. Accurately weigh 9.7 mg of hydrophobic pyrene fluorescent molecule, dissolve it in 10 mL of acetone, and then dilute to 4.6 × 10 -6 mol / L, spare. Into 3.6mL AGPR aqueous solution (take the ACBT-GGFLG-PEG prepared in step 2 of Example 2) that has prepared a series of concentrations 8 -R 8 GD powder was dissolved in deionized water) Add 45μL 4.6×10 -6 mol / L pyrene solution in acetone, configured as a sample. Place the prepared sample in a shaker (150r / min) at 37°C for 24 hours to ensure that the acetone is completely volatilized. Finally, the emission spectrum of the sample solution within 360-400 n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com