Slow-release soluble microneedle delivery system for mite-allergen-loaded microspheres
A delivery system and allergen technology, which is applied in the field of sustained-release soluble microneedle delivery system, can solve problems such as uncertainty, decline in mechanical properties of microneedles, and differences in mechanical properties of soluble microneedles, so as to prolong release time and reduce drug delivery. The effect of medication frequency and increased compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
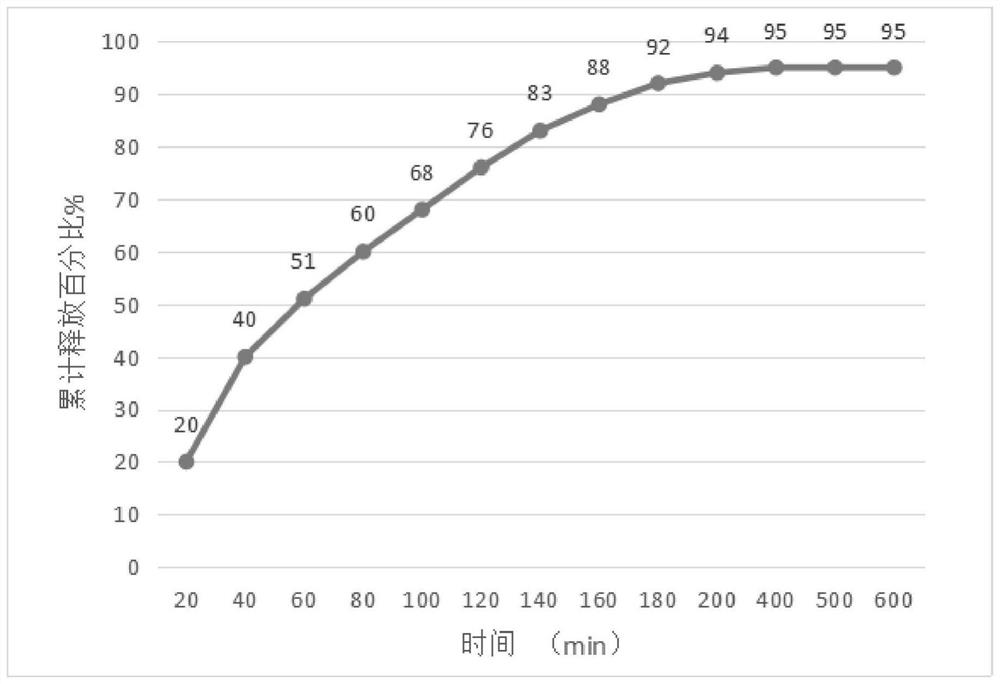
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of slow-release soluble microneedles for mite allergens
[0024] The mite allergen slow-release soluble microneedle to be prepared in the present invention is composed of mite allergen microspheres and microneedle matrix materials.
[0025] Among the many microsphere preparation methods, the double emulsion solvent evaporation method has the advantages of simple operation, convenient control of process parameters, no need to adjust pH and large temperature changes, and greatly improves the drug loading and encapsulation efficiency of microspheres compared with the single emulsion system. , has the advantages of controlled release and is often used to encapsulate water-soluble core materials. Therefore, the present invention adopts double emulsion solvent evaporation method to prepare mite allergen microspheres, and the specific method is as follows:
[0026] The capsule wall material and a small amount of Span 60 are dissolved in dichloromethane a...
Embodiment 2
[0031] Example 2 Related Determination of Mite Allergen Slow Release Soluble Microneedles
[0032] 1. Particle size of mite allergen microspheres
[0033] Precisely weigh the prepared dry microspheres and spread them evenly on the glass slide, observe the morphology with an optical microscope with a micrometer and count the particle size (μm) of 300 microspheres, divide the particle size range and calculate the average particle size through statistical processing. path.
[0034] When the average particle size of the microspheres is less than 30 μm, the microspheres are more likely to be distributed on the tip of the microneedle, which is considered to meet the requirements and can be used for subsequent preparations.
[0035] 2. Determination of Encapsulation Efficiency of Mite Allergen Microspheres
[0036] The total protein content of 10ml mite allergen vaccine solution was measured by Coomassie brilliant blue (CBB) method, and recorded as W1. Subsequently, the prepared mit...
Embodiment 3
[0048] Example 3 Effect of Component Types and Contents of Mite Allergen Microspheres on Mite Allergen Loading
[0049] In this example, the mite allergen microspheres consist of an oil phase, an inner water phase, and an outer water phase to form a three-phase system. The water phase is an aqueous solution of mite allergens, and the external water phase is a 0.5% concentration of polyvinyl alcohol (PVA) aqueous solution. By changing the type of cyst wall material, the volume ratio of the oil phase to the internal water phase, the results are shown in Table 1. Effects of mite allergen microspheres constructed of different materials on encapsulation efficiency and particle size.
[0050] In this embodiment, the capsule wall material is selected as polylactic acid-glycolic acid copolymer (PLGA), polyethylene glycol-polylactic acid acrylic acid copolymer (PEG-PLGA), polyethylene glycol-polylactic acid copolymer (PEG-PLA ), one of poly 3-hydroxybutyrate (PHB); the content ratio o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com