Probe for mass spectrometry
a mass spectrometry and probe technology, applied in the field of probes for mass spectrometry, can solve the problems of disfavorable radiolabels, limited current methods for analysing protein microarrays, and limitations in both methods, and achieve the effect of more accurate measuremen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0121] Affinity capture of a variety of tagged proteins can be demonstrated using for example PEG-PLL-biotin or PEG-PLL-Bleomycin B6 as the protein capturing moieties.
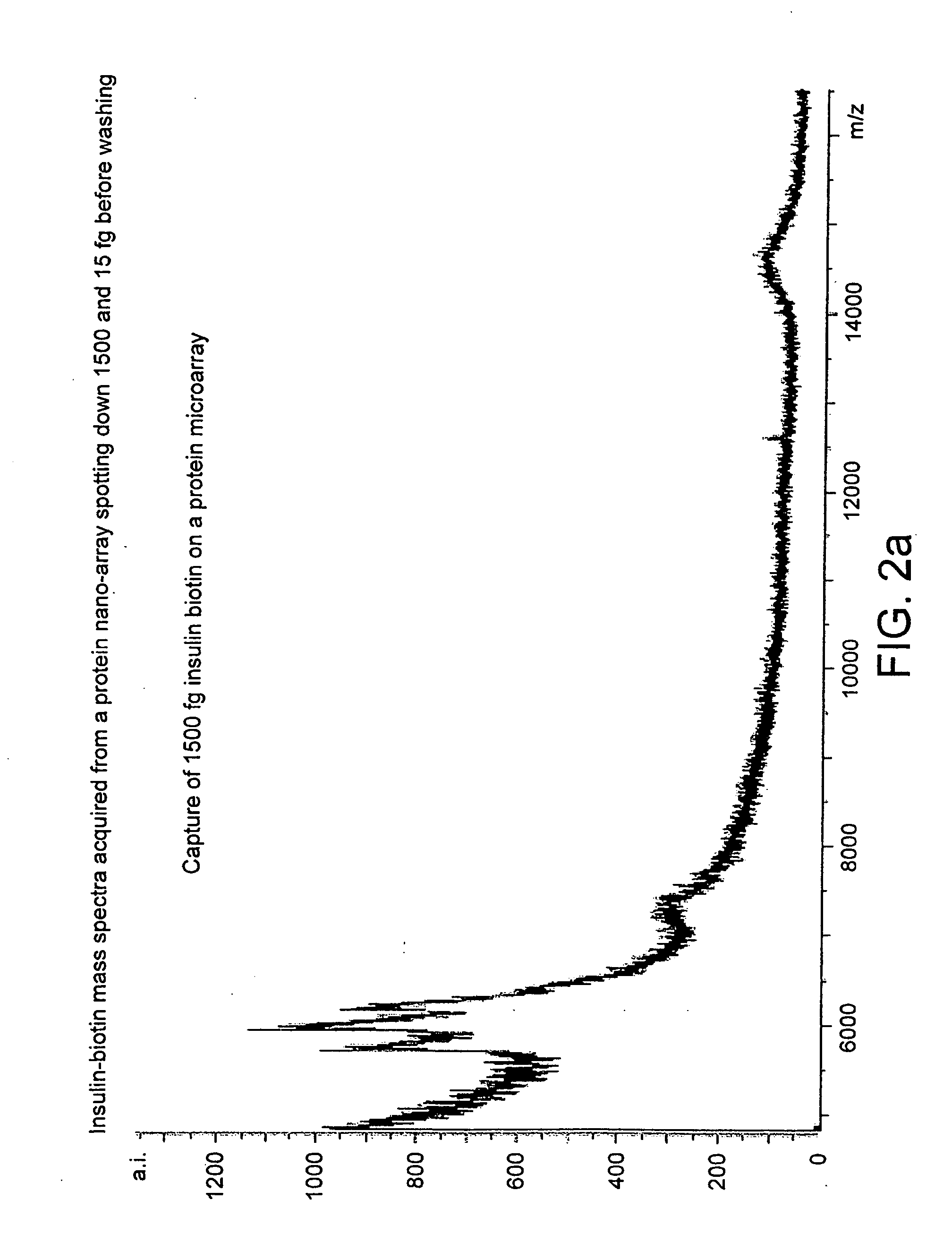
[0122]FIGS. 2, a and b show the mass spectra acquired from a protein microarray demonstrating respectively the capture of 1500 and 15 femtogram of biotin tagged insulin. The biotin tagged insulin was arrayed onto an affinity capture surface on a gold coated microscope glass slide in a 3 nanoliter volume using 300 micrometer pins (Q-Array, Genetix, New Milton, UK). The gold coated PEG-PLL-Biotin Neutravidin surface, was washed three times with 1 mM Tris-HCl pH 7.5, dried under a stream of nitrogen and overlaid with 3 nanolitre of α-cyano-4-hydroxy-cinnamic acid dissolved in 99% acetone v / v, 1% glycerol resulting in a spot with an radius of approximately 200 micrometer. The probe was analysed with a mass spectrometer MALDI TOF mass spectrometer. Several biotin tagged insulin peaks are visible due to the different degree...
example 2
[0124] A PLL-PEG-biotin neutravidin surface on a MALDI target is overlaid with 500 nanoliters of a biotinylated protein mixture derived from an E. coli lysate expressing a human recombinant protein in conjunction with a sequence tag in this case Biotin carboxyl carrier protein (BCCP) from E. coli. The protein was captured for a period of 2 hours on the surface, washed twice with washing buffer followed by two washes with desalting buffer, and overlaid with 300 nanoliters of an energy absorbing matrix, namely saturated α-cyano-4-hydroxycinnamic acid in acetone. The mass spectrum acquired in linear mode using the delayed extraction technique at low laser power is illustrated in FIG. 5. The advantage of this method is that the sample can be applied as a complex mixture of proteins and after washing only the molecules of interest remain. Secondly the analyte is captured in a spatially defined position before it is released from the affinity capture surface by the addition of matrix.
4....
example 3
[0125]FIG. 6 shows the peptide fingerprint analysis of Glutathione-S-transferase-Biotin Carboxyl Carrier Protein (GST-BCCP). A bacterial crude lysate containing the fusion protein and bacterial proteins was placed on the MALDI target previously coated with PEG-PLL-biotin and neutravidin. The BCCP fusion partner of GST contained a biotinylation consensus sequence such that it becomes biotinylated when expressed in E. coli. allowing the fusion protein to bind specifically to the PEG-PLL-biotin neutravidin surface, whilst allowing the bacterial proteins to be washed away with buffer. For identification purpose the surface captured protein was digested by overlaying it with trypsin and analysed by MALDI MS. A protein fingerprint analysis revealed 12 peptides belonged to GST from Schistosoma mansoni, 4 peptides belonging to Neutravidin and 3 to trypsin (see table 1), but no bacterial protein was identified using the remaining unmatched peptides. This experiment demonstrates that PEG-PLL-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com