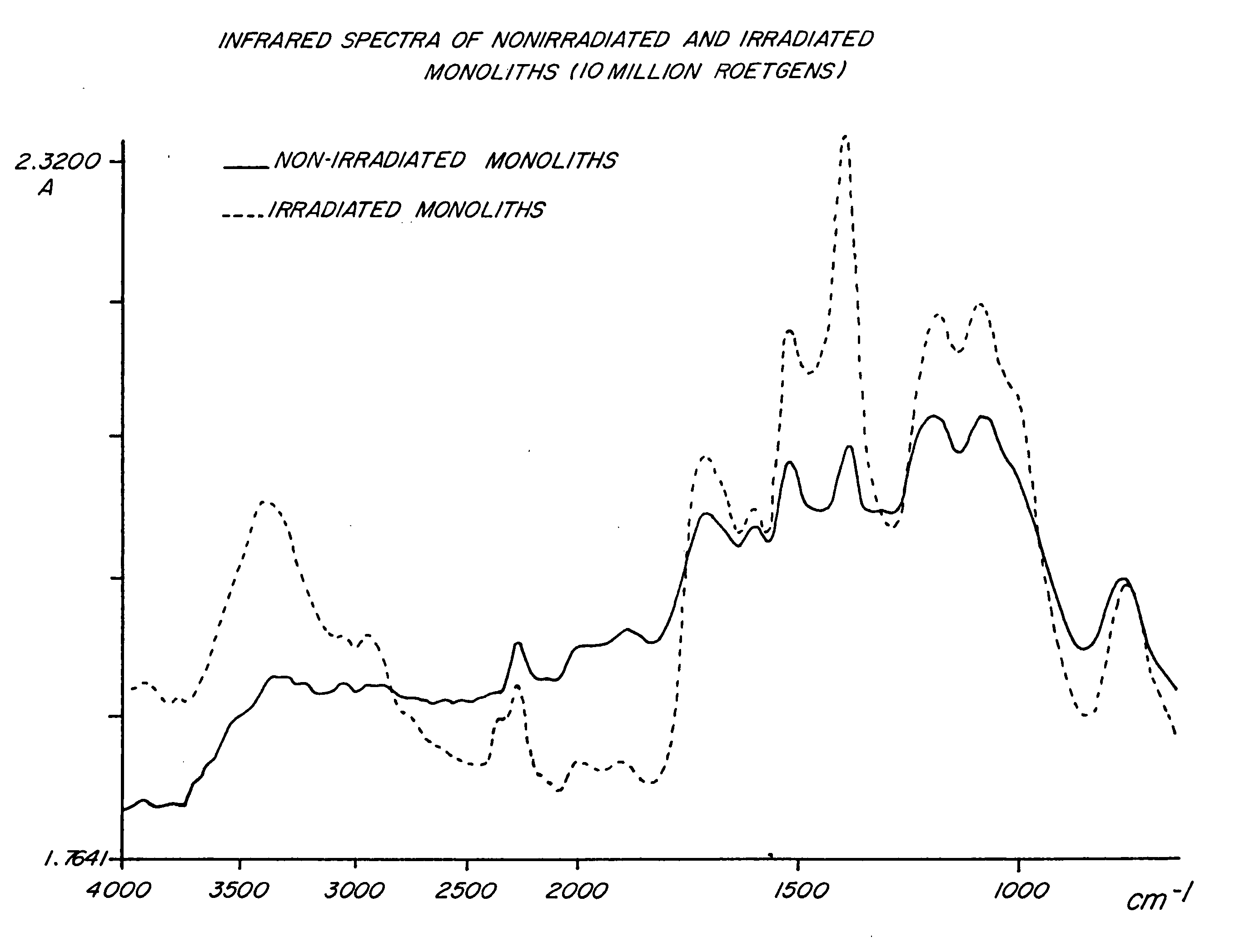
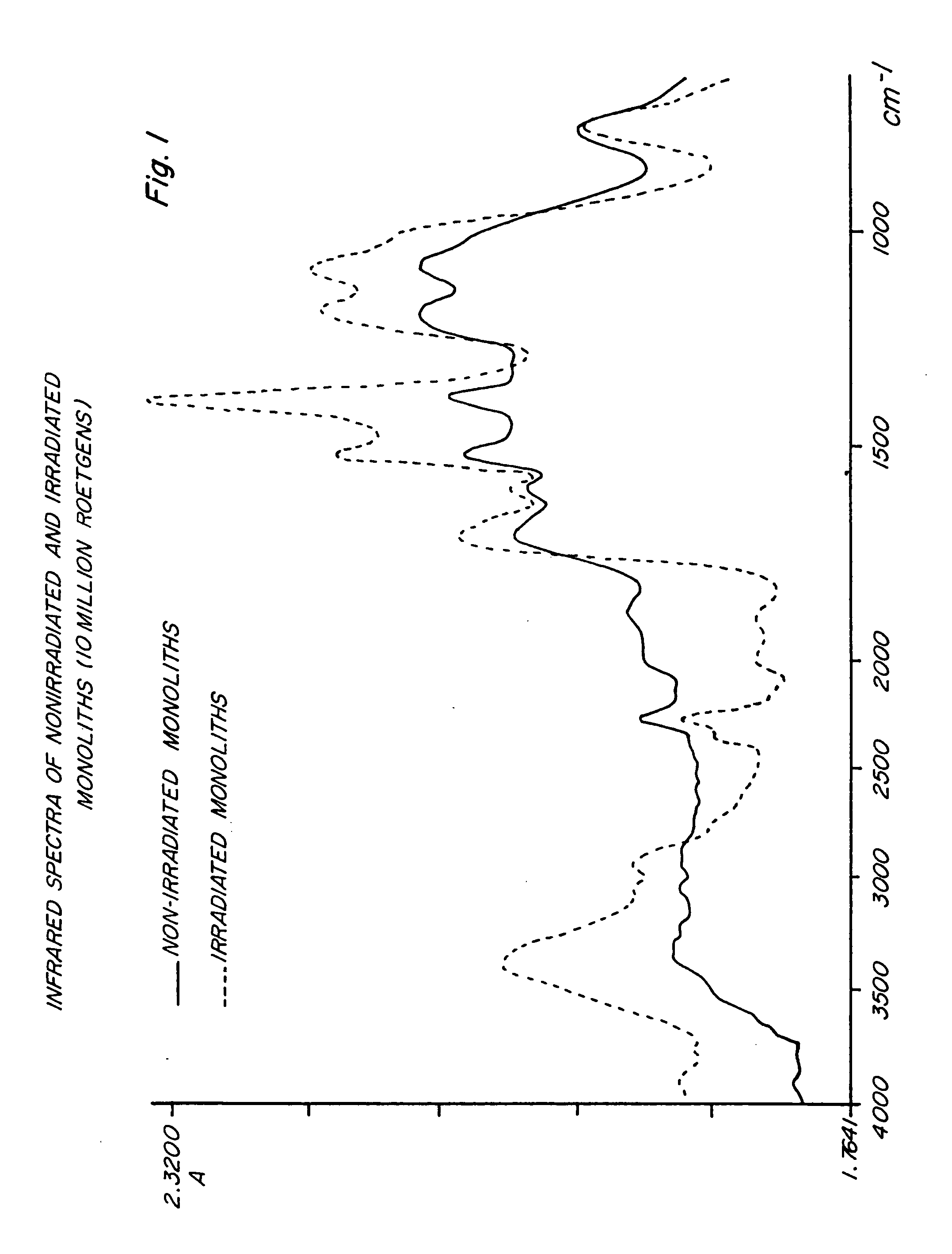
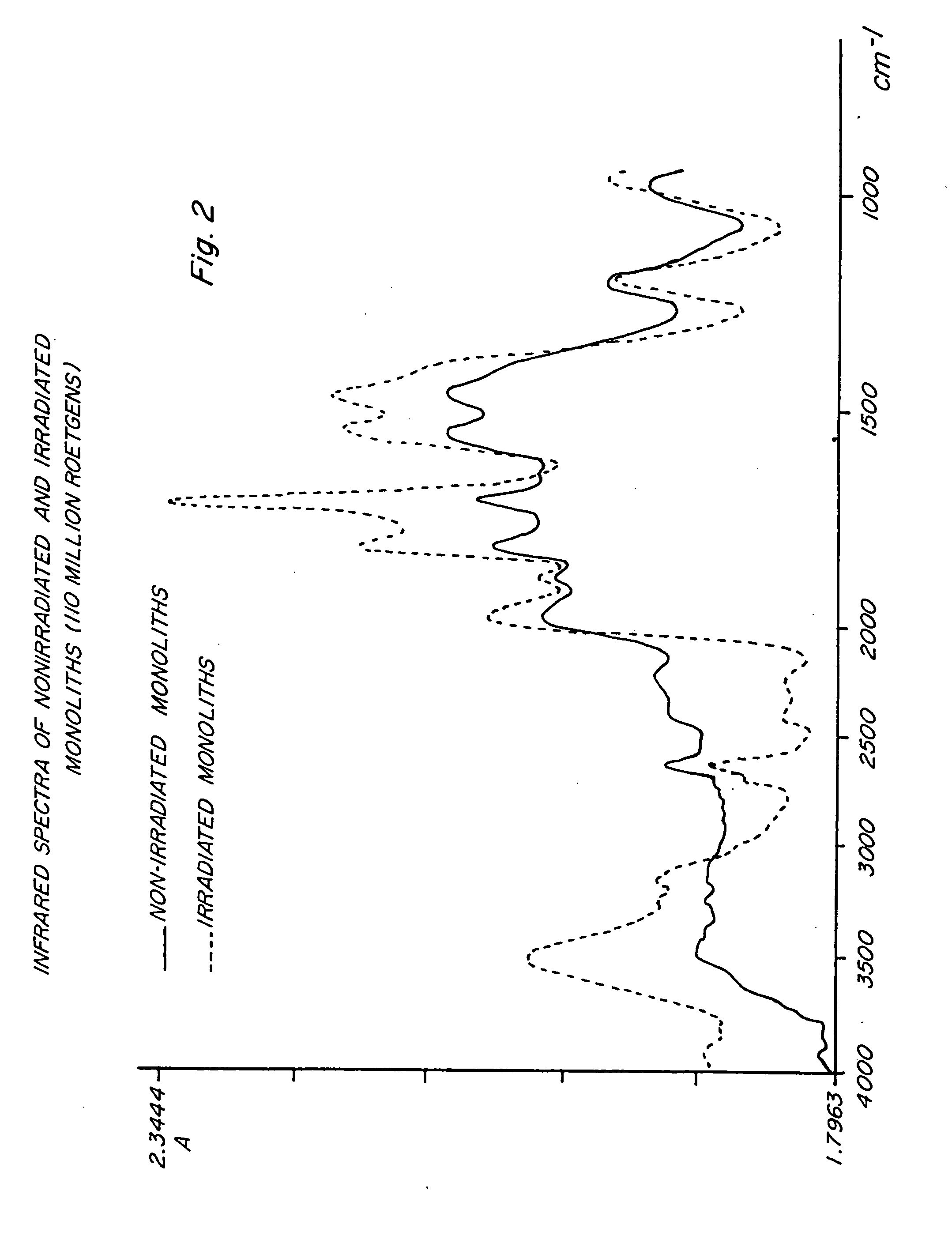
Polymer compositions and methods for shielding radioactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Polymer Composition
[0092]
Cas No.% by weightCOMPONENT APepset 1, HNS I by Ashland Chemical of Dublin, Ohio.Phenol Formaldehyde Resin9003-35-440.0-55.0Dioctyl Adipate103-23-110.0-20.0Phenol108-95-2 1.0-10.0Aromatic Petroleum Distillates64742-95-6 1.0-13.0Dimethyl Glutarate1119-40-0 7.0-22.0Dimethyl Adipate627-93-0 1.0-11.0Dimethyl Succinate106-65-0 1.0-11.01,2,4-Trimethylbenzene95-63-6 1.0-9.0COMPONENT BPepset 2, HNS II by Ashland Chemical of Dublin, Ohio.Polymeric MDI9016-87-930.0-40.0Methylene Diphenyldiisocyanate101-68-825.0-35.0Dioctyl Adipate103-23-1 1.0-9.0Trimethyl-1,3-Pentanediol Diisobutyrate6846-50-0 1.0-10.0Aliphatic Petroleum Distillates64742-47-8 1.0-10.0Aromatic Petroleum Distillates64742-40-5 1.0-5.0Methylene Diphenylisocyanate26447-40-5 1.0-5.01,2,4-Trimethylbenzene95-63-6 1.0-3.0COMPONENT Cphenylpropyl pyridine, Ashland Chemical of Dublin, Ohio.Pyridine Derivative2057-49-010.0-30.0Dimethyl Glutarate1119-40-040.0-60.0Dimethyl Adipate627-93-0 5.0-20.0Dimethyl Succinate...
example 2
Uranium Oxide / Polymer Composition
[0094] The polymer composition components A and B are as defined in Example 1. The components were mixed in a well polished stainless steel vessel with a Hobart paddle type mixing device, under a vented hood to protect the technician. Component A 3.0 kg and Component B 3.0 kg were added to the mixer, and mixed for approximately 60 minutes at 60-100 rpm until the two liquids were thoroughly mixed. A total of 96.0 kg uranium oxide in the forms of UO2, UO3 U3O8, and metallic Uranium was then added in increments of 5.0 g under continuous mixing until a homogeneous mixture was formed after about 60 minutes. The mixing took place in a well-ventilated area with air filtration, protective clothing, and gas masks for the workers.
[0095] Without the addition of a catalyst, the uranium oxide polymer composition cured after 18 hours. Prior to curing, the composition was a smooth dough-like material suitable for casting.
[0096] The material was formed into brick...
example 3
Metal Ion / Polymer Composition
[0108] A ten-kilogram quantity of clean Ottawa sand was spiked with a solution containing arsenic, cadmium, cobalt, chromium, copper, nickel, lead and zinc. The solution was produced by mixing nitrates of the metal ions (supplied by T. J. Baker) named above to yield a solution containing 1000 ppm of each metal ion. The solution was prepared with distilled deionized water. An aliquot of the solution was added to the sand resulted in a matrix containing 1000 ppm(mg / kg) of the metals listed. After oven drying at 105° C. the samples were analyzed using a Varian AA-1275 / GTA95 Spectrometer to confirm the presence of the metals. The following reagents were added to effect chemical fixation of the metals: [0109] 1. Sodium Phosphate (stoichiometric quantity +10% reagent) commercial grade-supplied by Fischer Scientific. [0110] 2. Sodium Sulfide (stoichiometric quantity +10% reagent) commercial grade-supplied by Fischer Scientific. [0111] 3. Ferric Chloride (stoic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com