Systems and methods using a solvent for the removal of lipids from fluids
a technology of fluid removal and solvent, applied in the direction of flow mixer, chemical/physical process, other medical devices, etc., can solve the problems of ischemia of the tissue supplied by the blood vessel, acute obstruction and ischemia of the distal blood vessel, and exposing fatty plaque deposits that may break away and embolize within the circulation, so as to reduce the amount of time needed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first example
(a) First Example
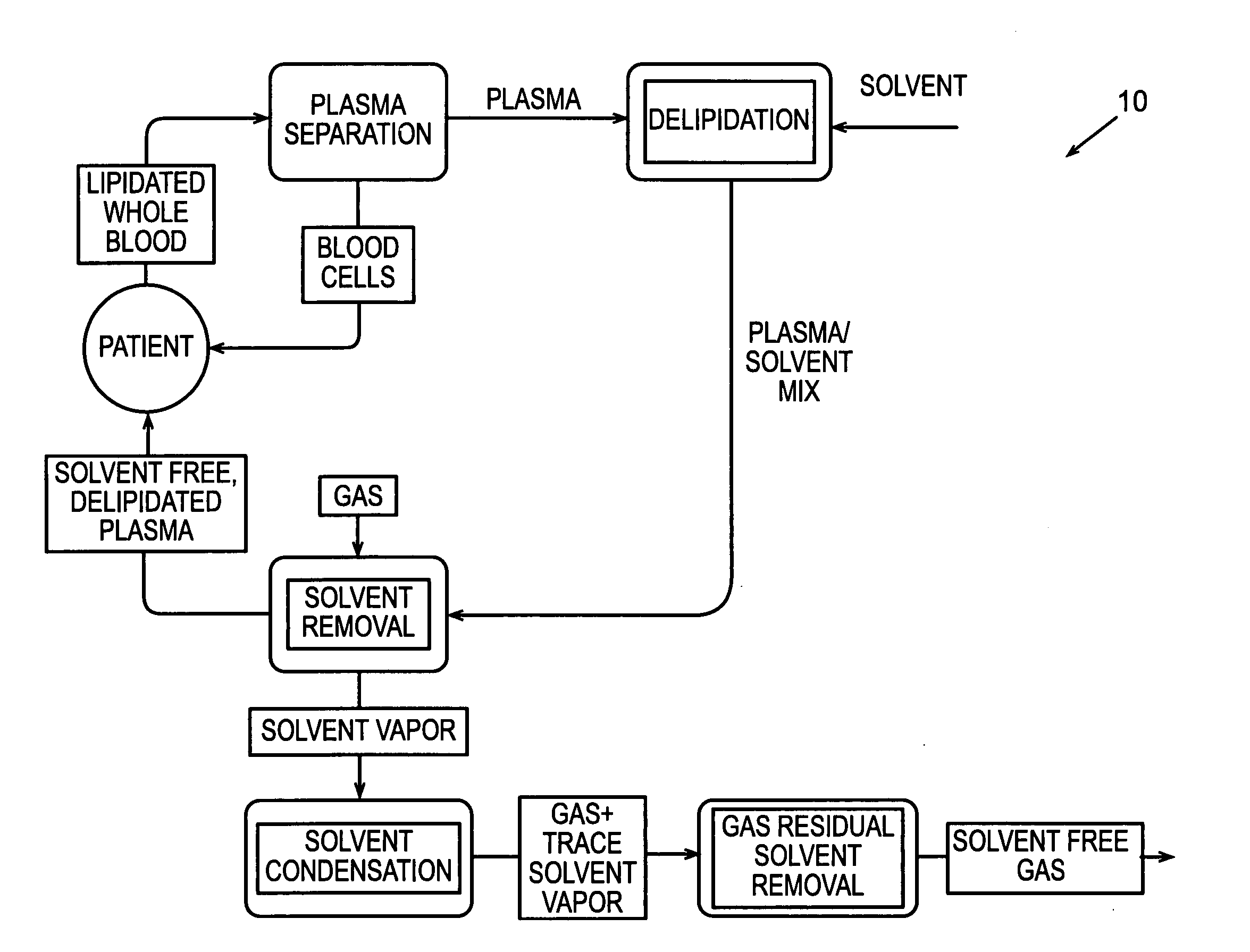
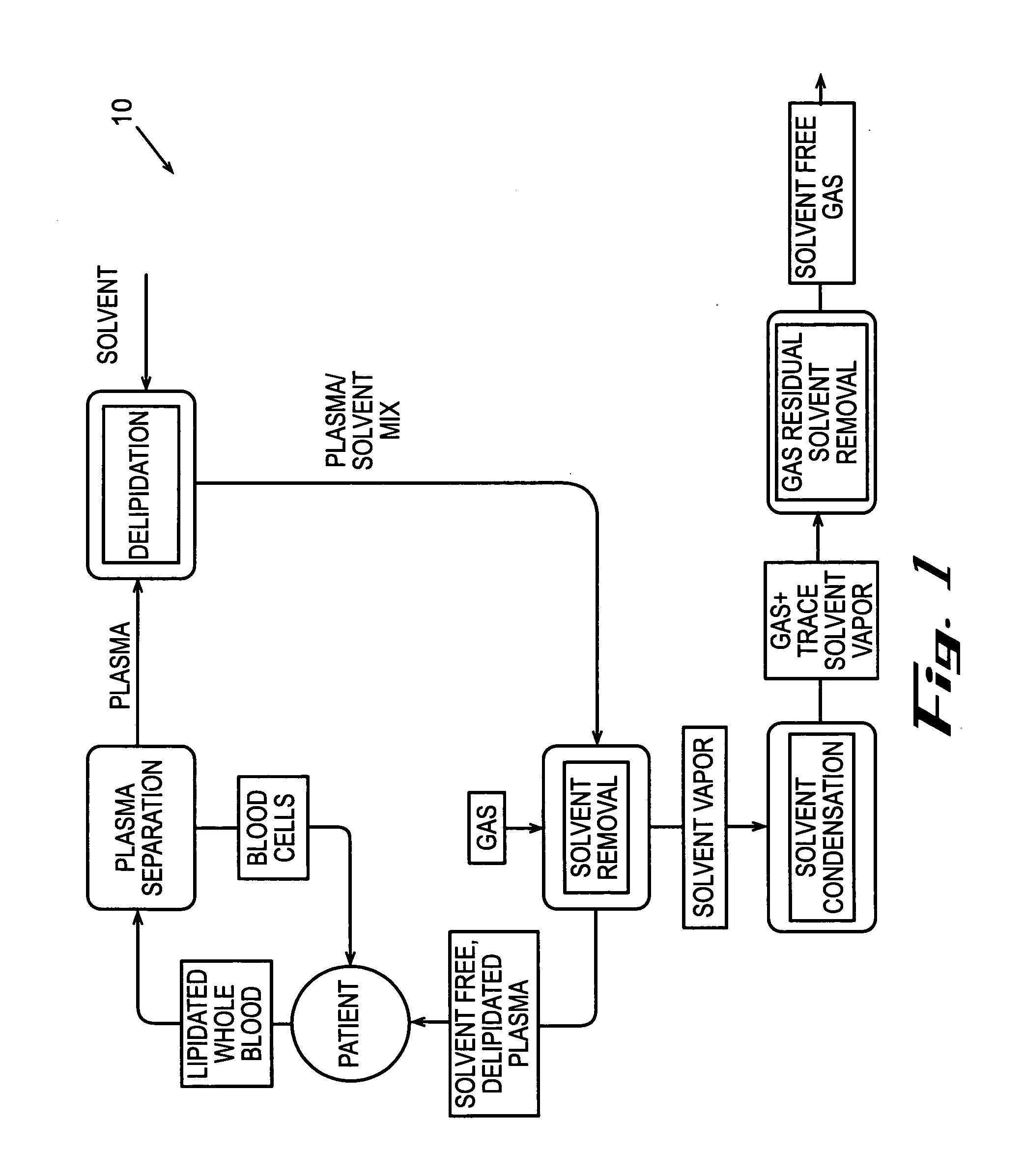
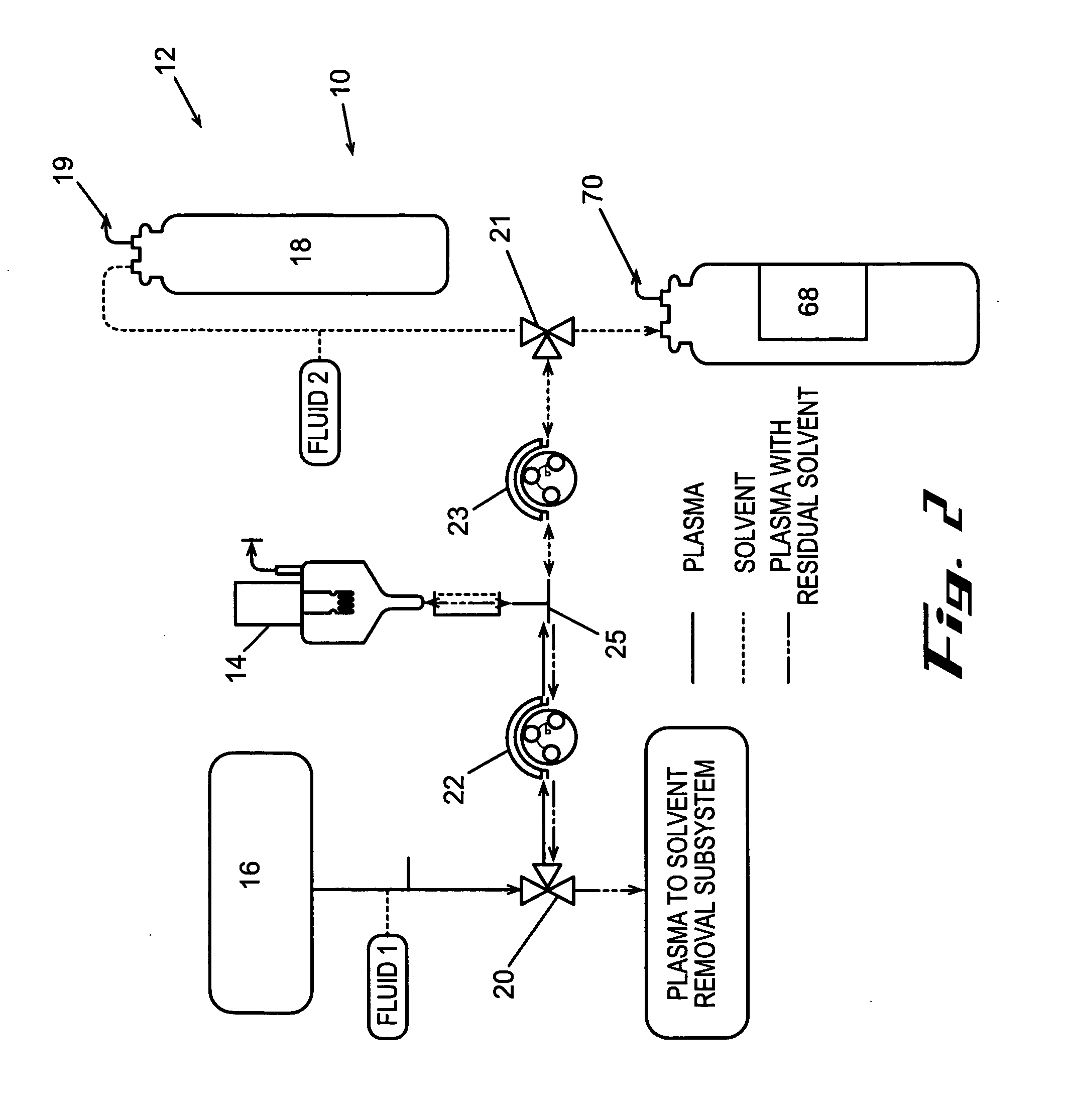
[0124] In accordance with the process described above and the embodiments, shown in FIGS. 2 and 3, human plasma was delipidated in an apparatus according to this invention by first introducing the plasma into a homogenizer with an equal volume of di-isopropyl ether (DIPE). The homogenizer used was a T25 UltraTurrax with a 25 mm diameter rotor head available from IKA Works of Germany. The homogenizer generated droplets having a diameter of about 5 um. The fluids were homogenized for about 6 minutes while the dispersion head rotated at about 24,000 rpm. The delipidated plasma containing residual delipidating solvent was then introduced into a solvent extraction device that resembled the subsystem shown in FIG. 17. The fluid was circulated through the hollow fibers of the HFCs at a flow rate of about 750 ml / min. Each HFC had a hold-up volume of about 50 ml and an area of about 4,800 cm2. Air was circulated through the shell of the HFC to extract the residual delipidati...
second example
(b) Second Example
[0126] This same apparatus was used to delipidate human plasma through numerous experiments. The speed of the homogenizer was varied between about 13,050 rpm and about 27,050 rpm and ran for between about one minute and about four minutes. This equated to an addition of 0.05 watts of energy per ml of solvent and fluid while running the homogenizer at about 13,050 rpm, and an addition of about 0.91 watts of energy per ml of solvent and fluid while running the homogenizer at about 27,050 rpm. The amounts of materials removed are the percentages of total concentrations of materials removed from initial concentrations of the materials in the fluid after running the homogenizer for about four minutes. The amount of cholesterol removed from the human plasma ranged between about 62.2% to about 91.5% for homogenizer speeds varied between about 13,050 rpm and about 27,050 rpm, respectively. The amount of triglycerides removed from the human plasma ranged between about 35.6%...
third example
(c) Third Example
[0128] Using a vortexer, as shown in FIGS. 7-9, human plasma was delipidated numerous times under various conditions. The amount of cholesterol, triglycerides, lipoprotein, phospholipids, apolipoprotein A1 and apolipoprotein B removed after adding 0.1 watts of energy per ml of fluid and solvent was about 30% after 10 minutes of running the vortexer, about 60% after 20 minutes, and about 90% after 30 minutes.
[0129] The percentages of constituents removed from the fluid differ when 1.0 watt of energy per milliliter of fluid and solvent was added using the vortexer. Specifically, the percentage of cholesterol removed from the fluid after one minute was about 67.3%, after two minutes was about 80.8%, after about 3 minutes was about 88.7%, after about 4 minutes was about 91.5%, and after about 8 minutes was about 95%. The percentage of triglycerides removed from the fluid after about one minute was about 53.1%, after about two minutes was about 64.6%, after about three ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com