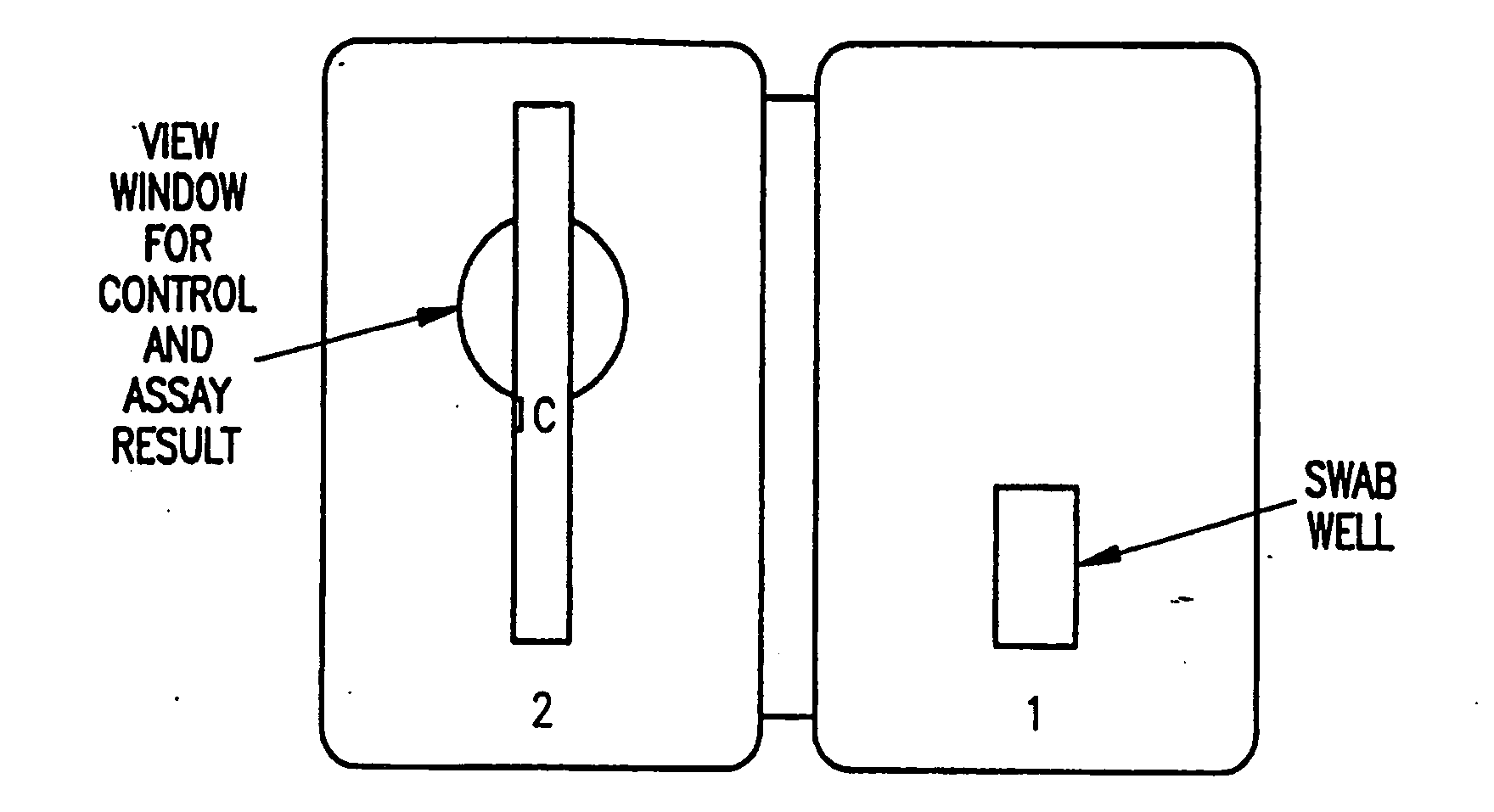
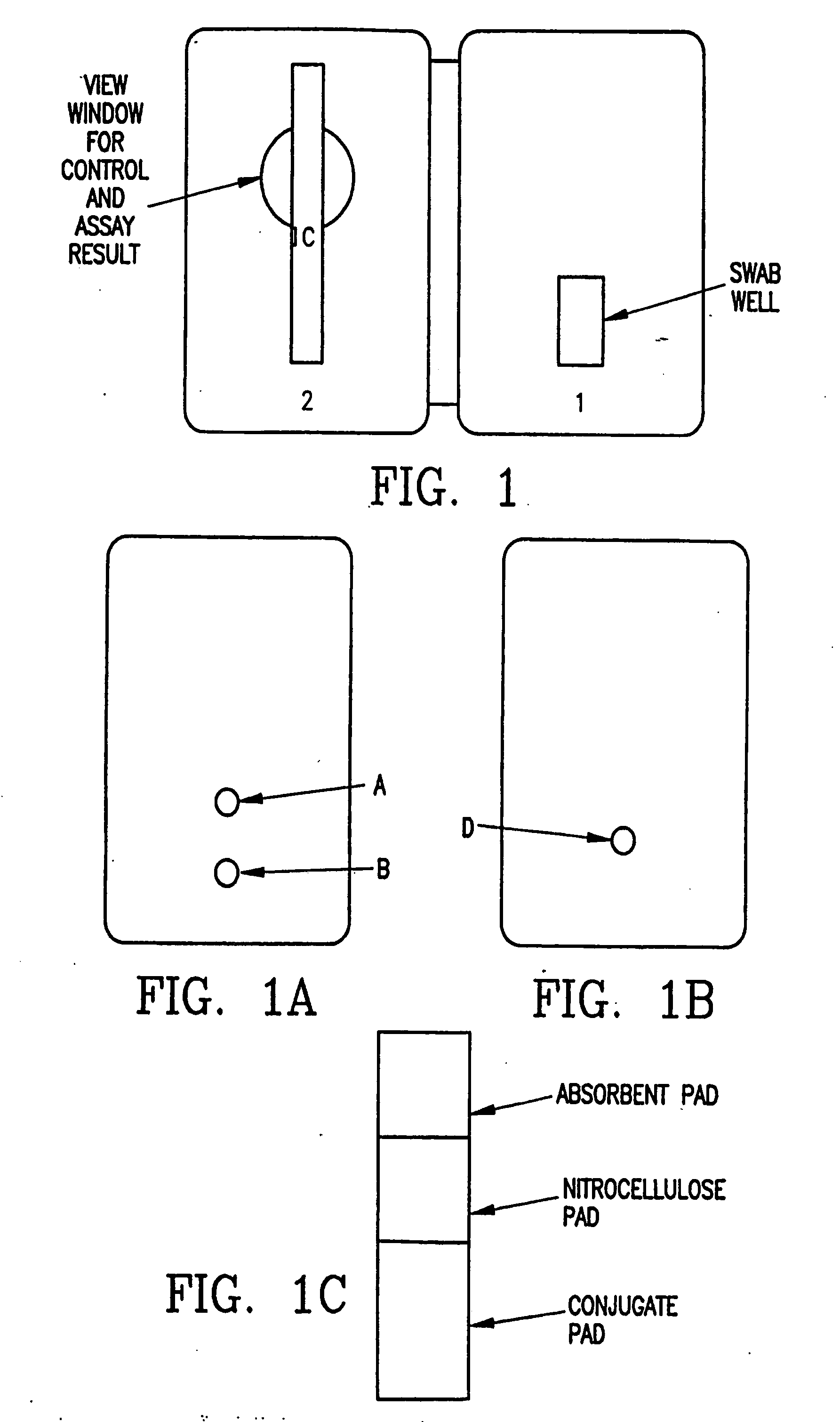
Process and materials for the rapid detection of streptococcus pneumoniae employing purified antigen-specific antibodies
a technology of streptococcus pneumoniae and purified antigens, applied in the direction of antibody medical ingredients, instruments, peptides, etc., can solve the problems of lack of sensitivity and/or specificity, time-consuming, labor-intensive, etc., and achieve rapid diagnosis of pneumonia, heightened risk of recurrence and subsequent death, and easy to perform
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example # 1
EXAMPLE #1
Bacterial Growth Conditions
[0031]S. pneumoniae strain R6 (ATCC No. 39938) was grown in S. pneumoniae broth supplemented with 20 mM of Hepes buffer. The broth had the following composition per liter:
Pancreatic digest of casein17.0g.Glucose10.0g.NaCl5.0g.Papain digest of soybean meal3.0g.Yeast extract3.0g.K2 HPO42.5g.HEPES20mM
This broth had an initial pH of 7.2±0.2 at 26° C. It was autoclaved for 15 minutes at 15 psi and 121° C. and set aside to cool.
[0032]Frozen aliquots of S. pneumoniae strain R6 (ATCC No. 39938) were inoculated onto 5% sheep blood agar plates and allowed to grow. Growth from the plates was harvested in smaller aliquots of the seed broth and this seed broth was inoculated into three flasks, each containing 1,700 ml of supplemented S. pneumoniae broth of the composition shown above and further grown at 37° C. in an atmosphere of 5 percent CO2, with agitation but not aeration. When the pH of the broth fell below 5.5 (its late log phase) the flasks were remov...
example # 2
EXAMPLE #2
Isolation of S. pneumoniae
C-Polysaccharide Antigen Containing Less than 10% Protein
[0033]Cells grown, treated and stored as in Example 1 were thawed at room temperature and suspended in phosphate-buffered saline solution (“PBS”) of pH 7.2 with 0.2 percent of sodium azide in a ratio of 1.2 ml. of buffer to 1 gram of wet cells and left at room temperature for two days.
[0034]Eleven ml per gram of the wet cells of 0.1 N NaOH was then added to the S. pneumoniae suspension (in phosphate buffered saline), resulting in a pH of 12.34 (as measured by pH meter) and incubated for 45 minutes at about 30° C. The pH of the suspension was then adjusted to 2.75 (measured by pH meter) with 2 N HCl, followed by centrifuging the suspension at 3,500 rpm for 25 minutes. The supernatant was then separated and its pH was adjusted to 7.0-7.1 with 1 N NaOH. This essentially neutralized supernatant was dialyzed at 4° C. against water for two days in dialysis tubing (obtained from Spectra / Por) havin...
example # 3
EXAMPLE #3
Preparation of BSA Conjugate of the Antigen
[0042]For coupling of the purified S. pneumoniae strain R6 C-polysaccharide antigen to a chromatographic column to permit affinity purification of rabbit anti-S. pneumoniae strain R6 antibodies, a BSA-hydrazine conjugate was selected. Other known materials having similar functions may be selected and conjugated to accomplish this coupling function.
[0043]The BSA-hydrazine conjugate was prepared as follows:
[0044]Hydrazine dihydrochloride obtained from Aldrich Chemical Co. was dissolved in water to produce an 0.5 M solution. The pH was adjusted to 5.2 with dry NaOH and dry bovine serum albumin (“BSA”) from Sigma Chemical Co. was added to produce a final concentration of BSA of 25 mg per ml of solution. After complete dissolution of BSA, N-(dimethylamino-propyl)-N1-ethylcarbodiimide hydrochloride (from Fluka Chemical Co.) was added in a quantity to produce a final concentration of 2.5 mg per ml of solution. This reaction mixture was i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| performance time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com