Preparation method of thin shell shaped noble metal catalyst
A precious metal catalyst and thin shell technology is applied in the field of preparation of thin shell precious metal catalysts, which can solve the problems of short life, high amount of precious metal, poor selectivity, etc., and achieve the effects of reducing the loss of raw materials, large specific surface area and reducing side reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
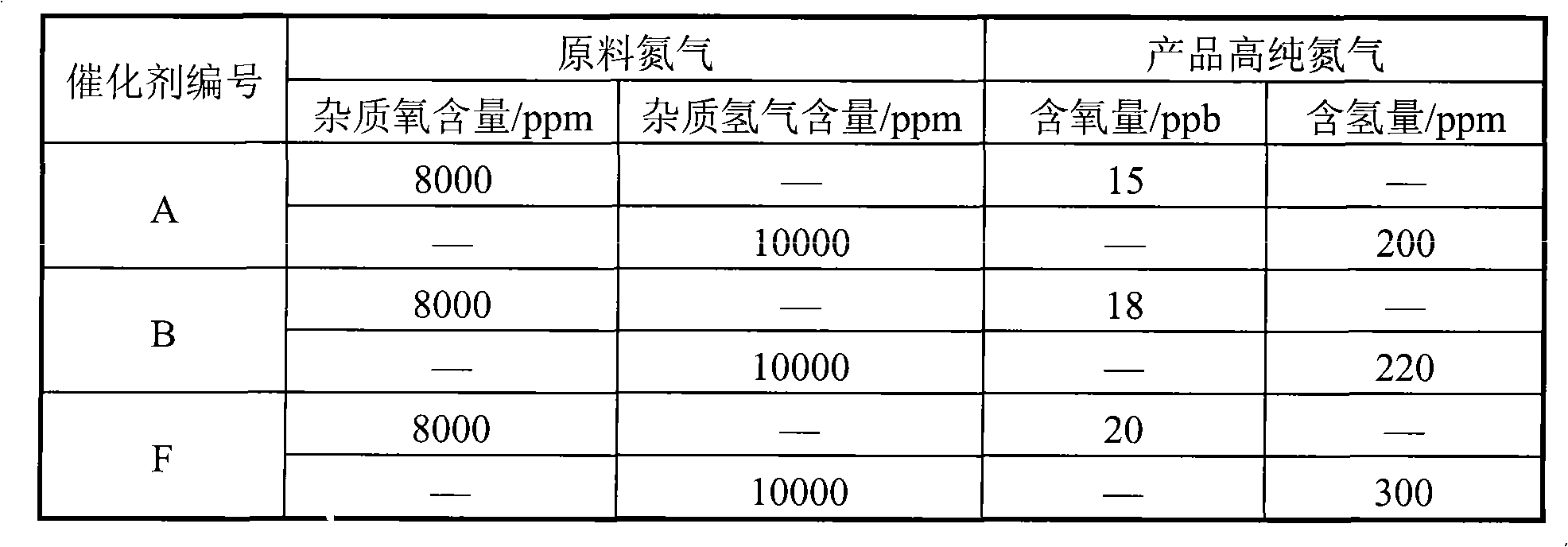
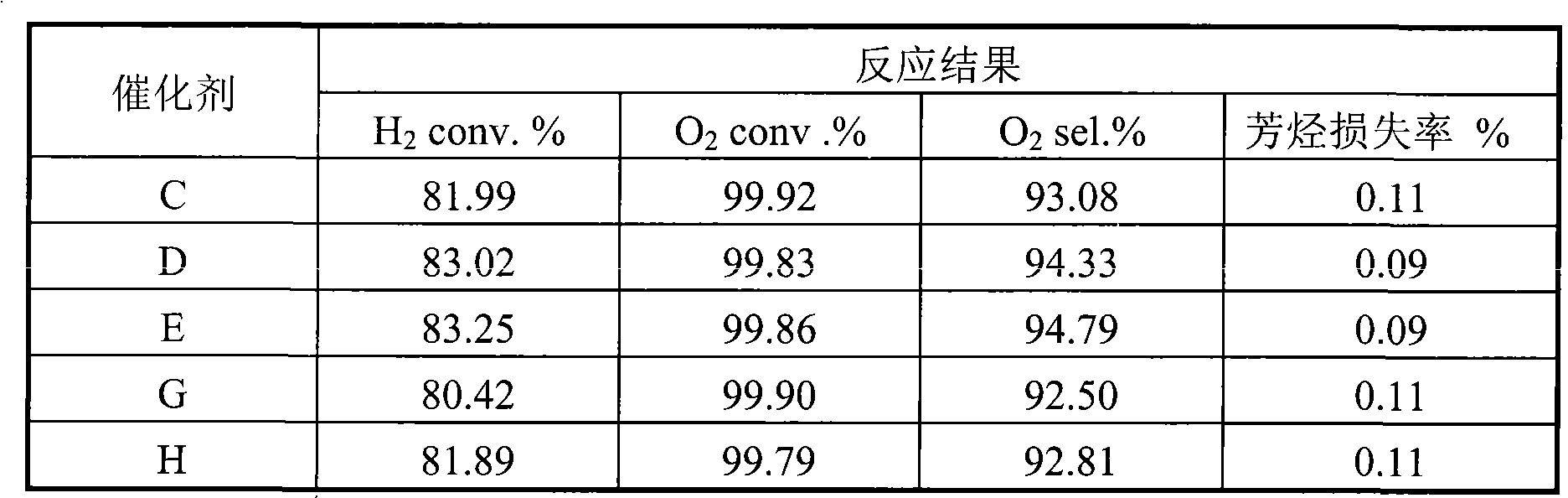
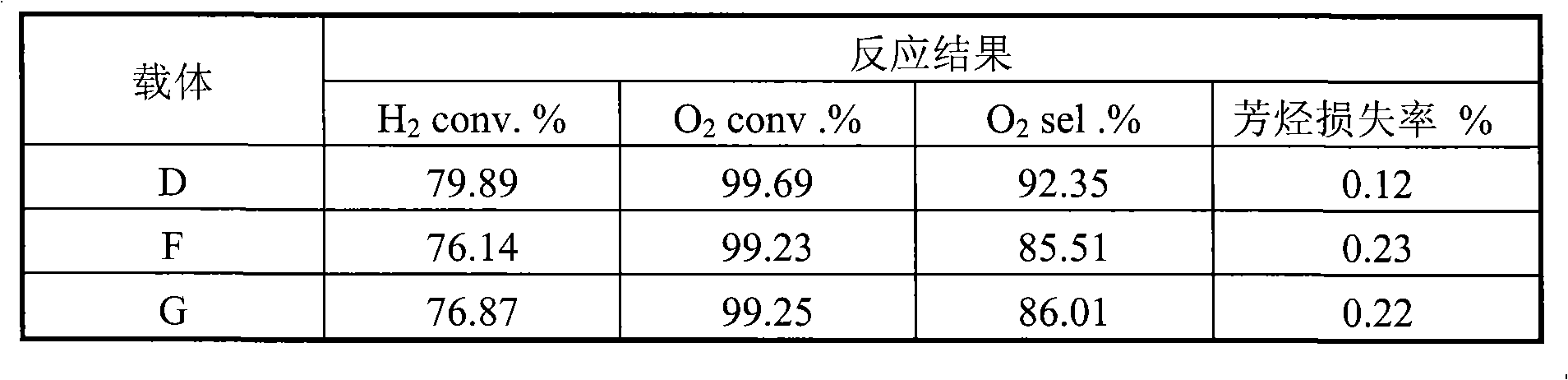
[0022] 20 grams of aluminum sol (containing 15% mass ratio of aluminum oxide), 3.0 grams of barium oxide, 0.6 grams of zirconium oxychloride, 50 grams of mordenite powder (below 5 microns in particle size, and a specific surface area of 270 meters 2 / gram), 12 grams of 6% polyvinyl alcohol and 100 grams of distilled water were mixed, stirred for 1.0 hour to prepare the coating slurry, and the cordierite pellets with a diameter of 4 mm were immersed in the prepared mixed coating slurry, and placed overnight , dried at 80°C for 2 hours, then heated up to 150°C and dried again for 2 hours, and finally calcined at 1100°C for 3 hours to obtain a layered composite carrier. Analysis shows that the thickness of the coating is about 120 microns, and the specific surface area of the coating is 178 meters 2 / gram.
[0023] Then the prepared composite layered support was immersed in chloroplatinic acid and palladium chloride solution with pH = 2-6, then dried at 120°C for 2 hours, fo...
Embodiment 2
[0025] 40 grams of alumina sol (containing 15% by mass ratio of alumina), 60 grams of 3% polyacrylamide solution, and 0.4 grams of betaine were prepared into slurry. Then add 0.3 grams of calcium silicate, 40 grams of θ-Al to this mixture 2 o 3 pink. After stirring for about ten minutes, add 2.0 g of 25% MgCl 2 As an aqueous solution, the resulting slurry was ball milled for 4 hours at room temperature to control the particle size below 20 microns. Slurry spraying to α-Al with a particle size of 4 mm 2 o 3 On the pellets, dry at 80°C for 2 hours, then heat up to 150°C and dry again for 2 hours, and finally bake at 800°C for 10 hours to obtain a layered composite carrier. Analysis shows that the thickness of the coating is about 80 microns, and the specific surface area of the coating is 138 meters 2 / gram.
[0026] The layered composite support was impregnated in a mixed solution of lithium nitrate and palladium chloride by an equal volume impregnation method in a rot...
Embodiment 3
[0028] 35 grams of alumina sol (containing 25% mass ratio of alumina), 5 grams of 40% silica sol, 60 grams of 4% cyclodextrin solution, 2.0 grams of lanthanum oxide, 1.0 grams of hexadecyl trimethyl bromide ammonium chloride to make a slurry. Then add 0.4 grams of calcium silicate, 0.3 grams of potassium carbonate and 40 grams of pre-treated δ-Al with a size below 10 microns to this mixture 2 o 3 pink. The resulting slurry was ball milled for 4 hours at room temperature to control the particle size below 10 microns. The slurry was sprayed onto mullite pellets with a particle size of 4 mm, dried at 100°C for 2 hours, then heated to 150°C for another 2 hours, and finally calcined at 900°C for 6 hours to obtain a layered composite carrier. Analysis shows that the thickness of the coating is about 100 microns, and the specific surface area of the coating is 120 meters 2 / gram.
[0029] Measure 0.16 ml concentration of 50wt% SnCl 4 solution, add water to 16 ml. Weigh out 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com