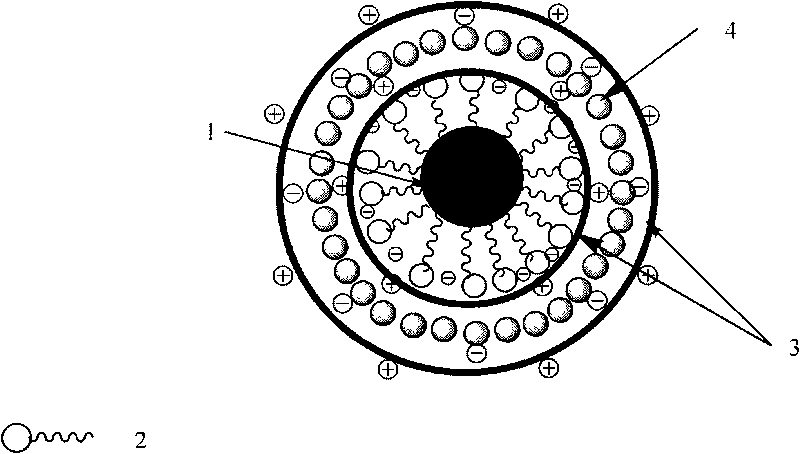
Polysaccharide/inorganic nanoparticles hybrid micron-nano medicine-carrying capsule
A micro-nano and polysaccharide technology, applied in micro-capsules, capsule delivery, inorganic inactive ingredients, etc., can solve the problems of low drug loading rate, use, and difficulty, and achieve high-efficiency drug in-situ encapsulation, good biological Compatibility and biodegradability, the effect of good biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: take elemene, OSA modified starch sodium solution as an example to illustrate
[0034] At normal temperature, the fat-soluble drug elemene is added to the 5wt% OSA modified starch aqueous solution, the concentration is controlled at 5wt%, vigorously mechanically stirred under the condition of 15000-18000rpm, and then the above-mentioned crude emulsion is added into the high-pressure homogenizer ( 100bar) homogenized 4 times to obtain nanoemulsion.
Embodiment 2
[0035] Embodiment 2: Take paclitaxel / medium chain triglyceride, OSA modified starch sodium solution as an example for illustration
[0036] 5 g of paclitaxel was dissolved in 50 ml of medium-chain triglyceride (Miglyol 812, Shanghai Sinopharm Chemical Reagent Co., Ltd.), and stirred thoroughly to obtain a 10 wt% (w / w) paclitaxel oil phase solution. At room temperature, add 50mL of 10wt% (w / w) paclitaxel solution prepared into 50mL of OSA modified starch (5wt%) aqueous medium, vigorously stir mechanically at 15000-18000rpm, then add the above crude emulsion Homogenize 4 times in a high-pressure homogenizer (100 bar) to obtain a nanoemulsion.
Embodiment 3
[0037] Embodiment 3: illustrate with zedoary oil, OSA modified starch sodium solution.
[0038] At normal temperature, zedoary oil was added to OSA modified starch (5wt%) aqueous medium, the concentration was controlled at 10wt%, vigorously mechanically stirred under the condition of 15000~18000rpm, then the above-mentioned coarse emulsion was added in the high-pressure homogenizer ( 100bar) homogenized 4 times to obtain nanoemulsion.
[0039] Two, the preparation of capsule: take the oil phase droplet in the nanoemulsion as soft template, take one or more in chitosan, 2-hydroxypropyl trimethyl chitosan quaternary ammonium salt as polycation, with Sodium alginate, λ-carrageenan, Fe with negatively charged surface 3 o 4 / Fe 2 o 3 One or more of the magnetic nanoparticles and the Au nanoparticles are polyanions, and the hybrid micro-nano drug-loaded capsules are formed under high-pressure homogeneous conditions through layer-by-layer self-assembly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com