A kind of preparation method of conductive ink nano copper
A technology of conductive ink and nano-copper, which is applied in the direction of ink, nanotechnology, household appliances, etc., can solve the problems of high raw material ratio and purity requirements, low reaction temperature, narrow particle size distribution, etc., and it is easy to achieve particle size and shape Control and reaction conditions are simple and mild, and the effect of high monodispersity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 0.20g of copper hydroxide to 40ml of ethanol, add 20ml of PEG-200, stir well and heat to 60°C to obtain solution a; add 3.52g of L-ascorbic acid to 40ml of ethanol, stir well and heat to 60°C to obtain solution b; add solution b to solution a, continue to stir for 5 minutes, then cool to room temperature, centrifuge and wash 4 times with 3000r / min deionized water, take the precipitate and dry it at room temperature for 6 hours under the condition of vacuum degree less than 0.01MPa, to obtain conductive ink nano-copper;
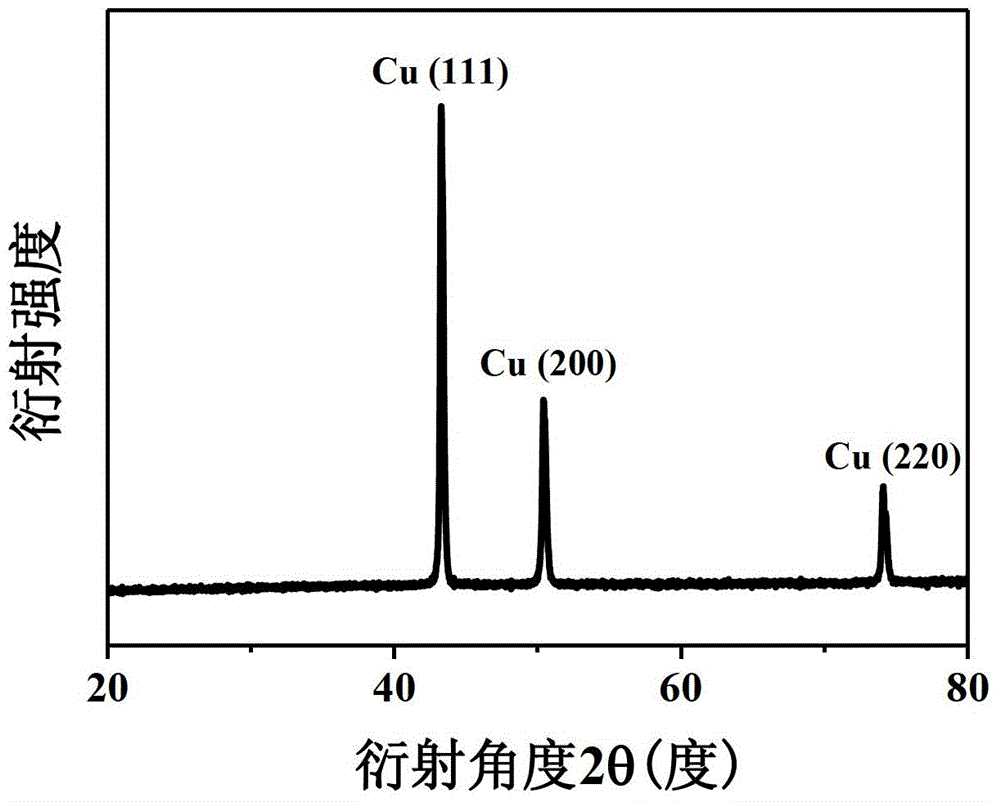
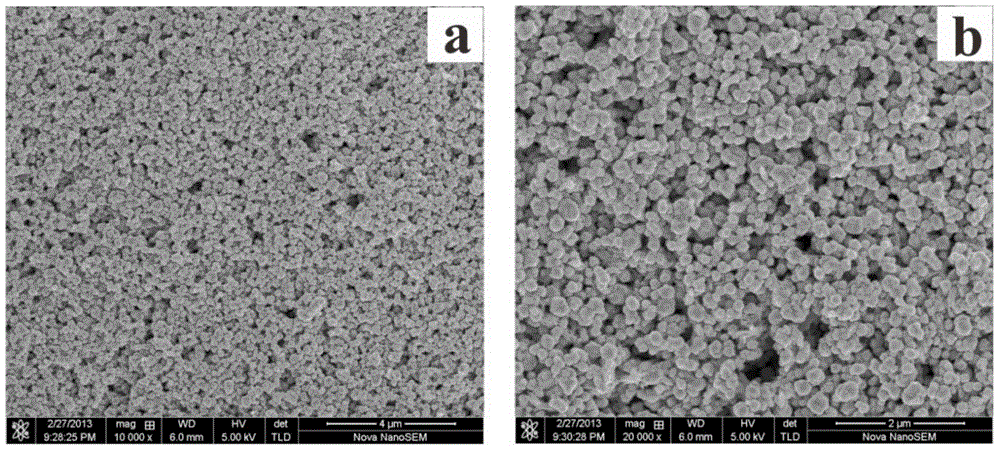
[0028] Take the above-mentioned nano-copper, use X-ray diffractometer (XRD) to analyze the phase pattern of the particles, and use the field emission scanning electron microscope (SEM) to observe the morphology of the particles, the results are as follows figure 1 and figure 2 shown. From figure 1 The characteristic peaks of copper can be seen in , without any characteristic peaks of the second phase impurities such as cuprous oxide or copper oxi...
Embodiment 2
[0030] Add 0.64g of copper sulfate to 40ml of ethylene glycol, add 15ml of PEG-400, stir well and heat to 80°C to obtain solution a; add 4.23g of L-ascorbic acid to 40ml of ethylene glycol, stir well and heat to 80°C , to obtain solution b; add solution b to solution a, continue to stir for 30 minutes, then cool to room temperature, centrifuge and wash 4 times with 5000r / min absolute ethanol, take the precipitate and dry it at room temperature for 6 hours under the condition that the vacuum degree is less than 0.01MPa to obtain conductive ink Nano copper;
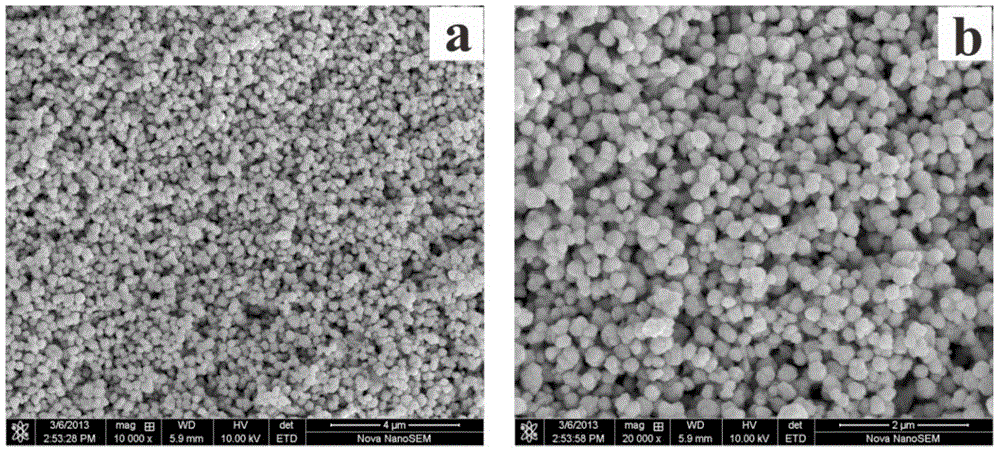
[0031] The XRD pattern and SEM pattern of the prepared nano-copper are similar to those in Example 1, indicating that the phase of the nano-copper is pure copper without other second-phase impurities; the particles have good dispersion and no obvious agglomeration.
Embodiment 3
[0033] Add 2.09g of copper acetylacetonate into 40ml of diethylene glycol, add 10ml of PEG-600, stir evenly and heat to 100°C to obtain solution a; add 4.93g of L-ascorbic acid into 40ml of diethylene glycol, Stir evenly and heat to 100°C to obtain solution b; add solution b to solution a, continue to stir for 1 hour, then cool to room temperature, centrifuge and wash 4 times with acetone at 8000r / min, take the precipitate and dry it at room temperature under the condition of vacuum degree less than 0.01MPa 6h, obtain conductive ink nano-copper;
[0034] The XRD pattern and SEM pattern of the prepared nano-copper are similar to those in Example 1, indicating that the phase of the nano-copper is pure copper without other second-phase impurities; the particles have good dispersion and no obvious agglomeration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com