Glucose-like peptide and preparation method thereof as well as injectable hydrogel
A hydrogel and injectable technology, which is applied in pharmaceutical formulations, medical preparations of non-active ingredients, medical science, etc., can solve the problems of hydrogels that do not have biological activity, are difficult to modify, and have poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The present invention also provides a method for preparing the above-mentioned glycopeptides with the structure of formula (I), comprising: adding one or more of the compounds with the structures shown in formula (V-1) to formula (V-3) 1. The compound with the structure of formula (IV) and the compound with the structure of formula (VI) are mixed with an organic solvent, and reacted under the action of a catalyst to obtain the glycopeptide with the structure of formula (I).
[0057]
[0058]
[0059] Among them, x, y, z and n are the degree of polymerization; 0≤x≤300; 15≤y≤800; 3≤z≤200; and 30≤x+y+z≤1000; n=x+y+z; The x, y, and z are the same as those described above, and will not be repeated here.
[0060] -R 1 It has one of the structures shown in the following formula (II-1) ~ formula (II-3):
[0061]
[0062] -R 2 It has the structure shown in the following formula (III):
[0063]
[0064] The present invention does not limit the sources of all raw m...
Embodiment 1
[0086] 1.1 In an ice-water bath, mix 20g of L-glutamic acid and 30ml of propynyl alcohol, mix and add 8ml of concentrated sulfuric acid dropwise under stirring conditions, after the dropwise addition, react at room temperature for 12-24h; after the reaction, add 27g of sodium bicarbonate aqueous solution Neutralize the reaction, filter and drain to obtain a crude product, which is recrystallized with 150ml of water to obtain γ-propargyl-L-glutamate.
[0087] 1.2 Add 100ml of dried tetrahydrofuran to a dry reaction flask, and continue to add 7.4g of γ-propargyl-L-glutamate obtained in 1.1 and 4g of triphosgene under a nitrogen atmosphere, and react at 55°C for 0.5 ~1h, after the reaction solution is clarified, stir at room temperature for 30min, then precipitate with cold petroleum ether; pour off the supernatant, dissolve the viscous substance in the lower layer with ethyl acetate, wash with cold water three times, and dry the organic phase with anhydrous magnesium sulfate over...
Embodiment 2
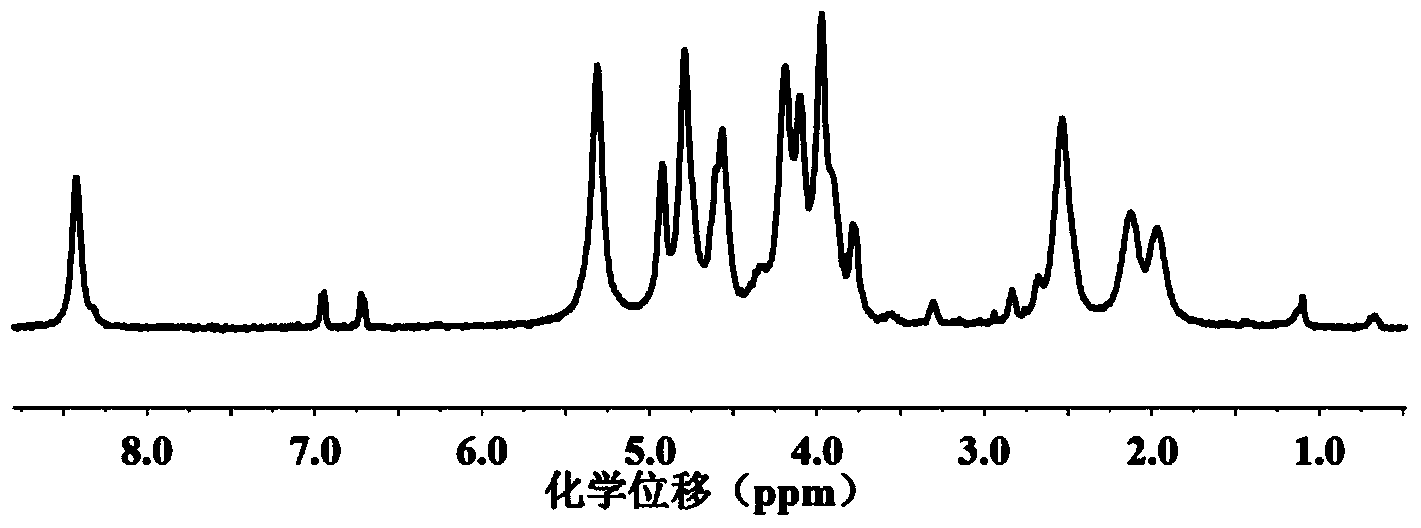
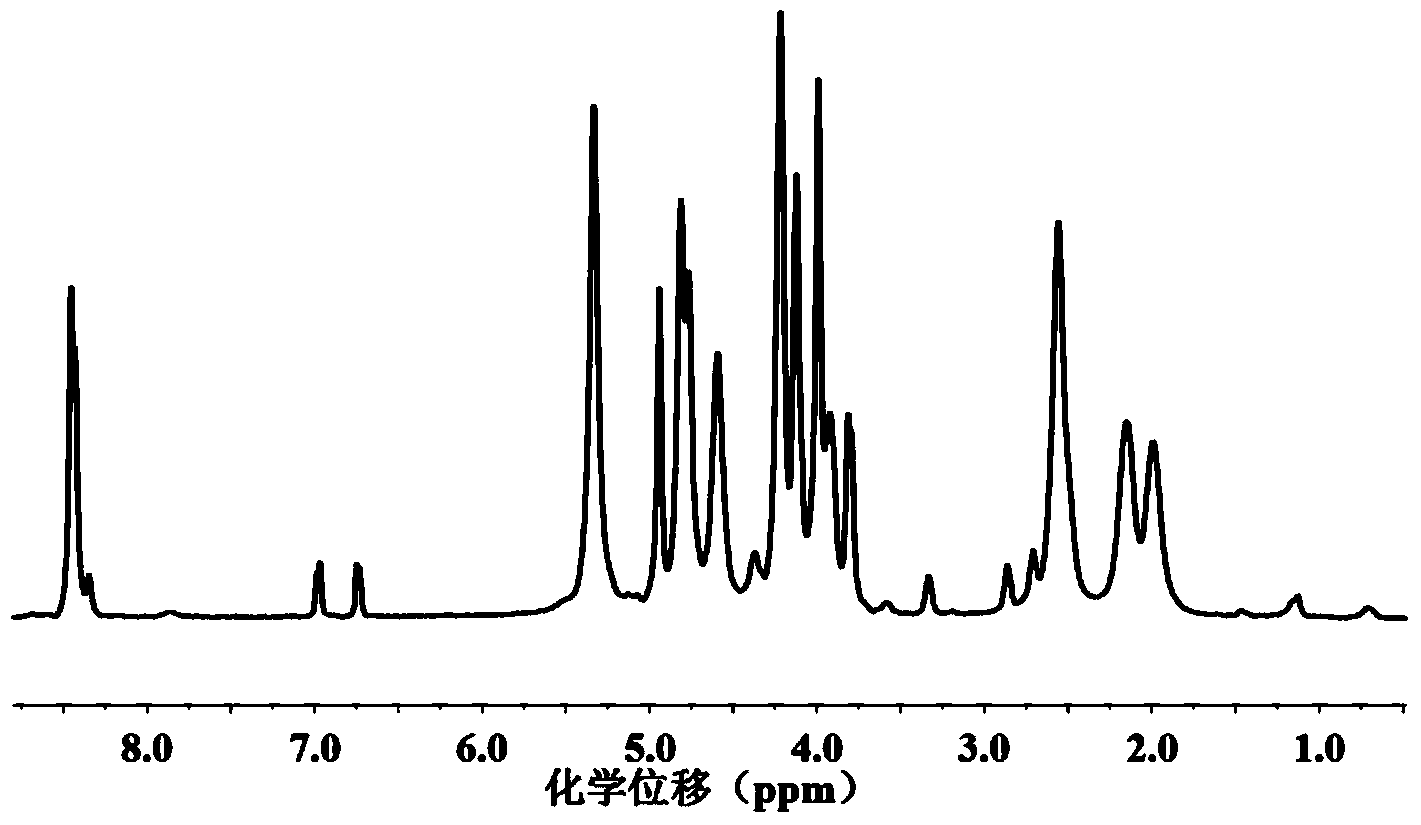
[0090] 2.1 Mix 2g of the compound of formula (IV) obtained in Example 1, 6.0g of the compound of formula (V-1) 2-azidoethyl-O-α-D-mannose with 0.15g of the compound of formula The compound of structure (VI) N-(3-azidopropyl) p-hydroxyphenylpropanamide was dissolved in 40ml of dimethyl sulfoxide, then bubbled with nitrogen for 30min to remove dissolved oxygen, and then added 0.3g of cuprous bromide With 0.5ml of pentamethyldivinyldiamine, continue bubbling for 5min, seal it, and react under nitrogen atmosphere at 25°C for three days. Distilled water was dialyzed for three days, and the glycopeptide with the structure of formula (I) was obtained after freeze-drying.
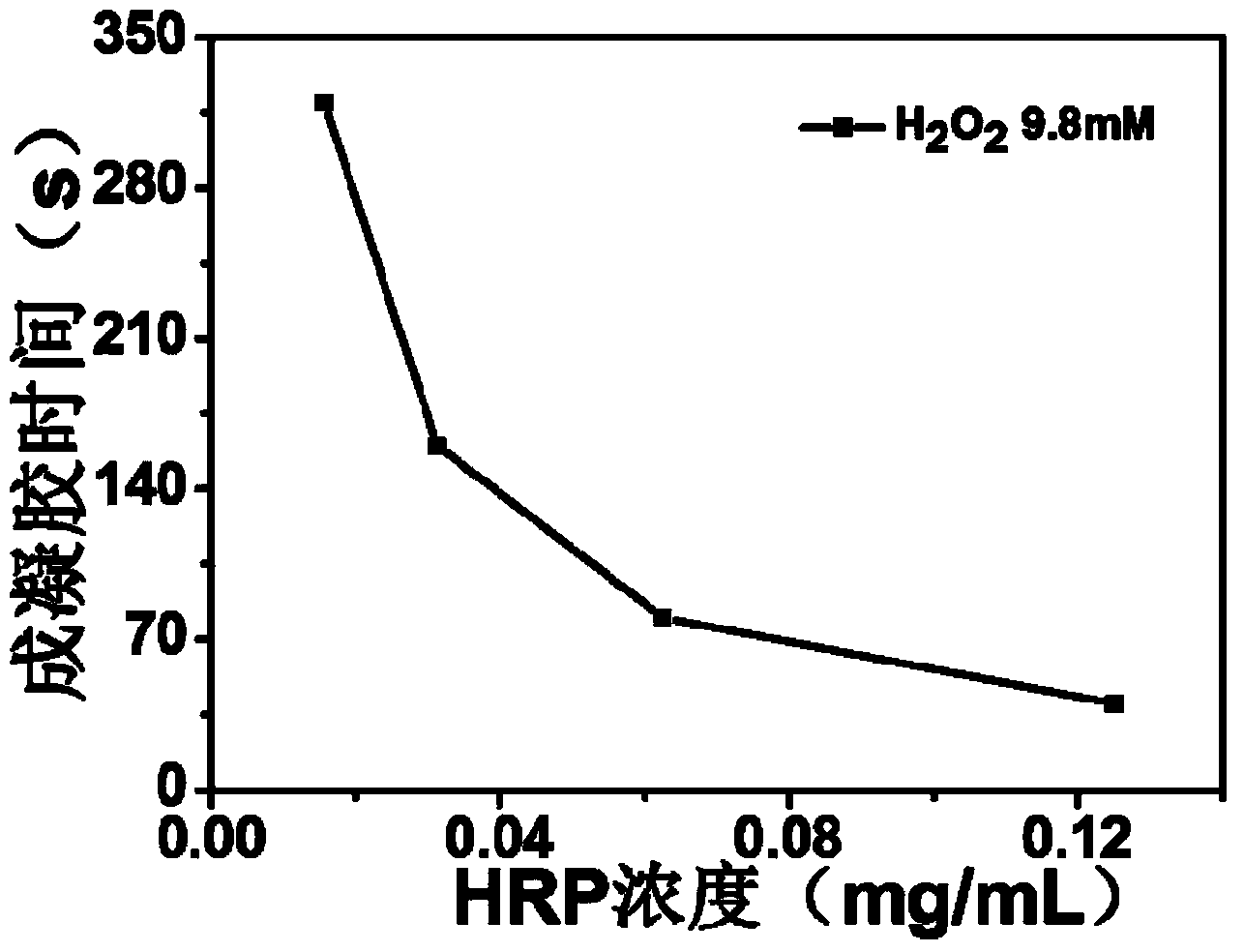
[0091] 2.2 Prepare the glycopeptide with the structure of formula (I) obtained in 2.1 into a phosphate buffer solution with a mass concentration of 6%-20% as the first mixed solution; prepare hydrogen peroxide into 2.45-49mmol / L phosphoric acid The salt buffer solution is the third mixed solution; the phosphate bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com