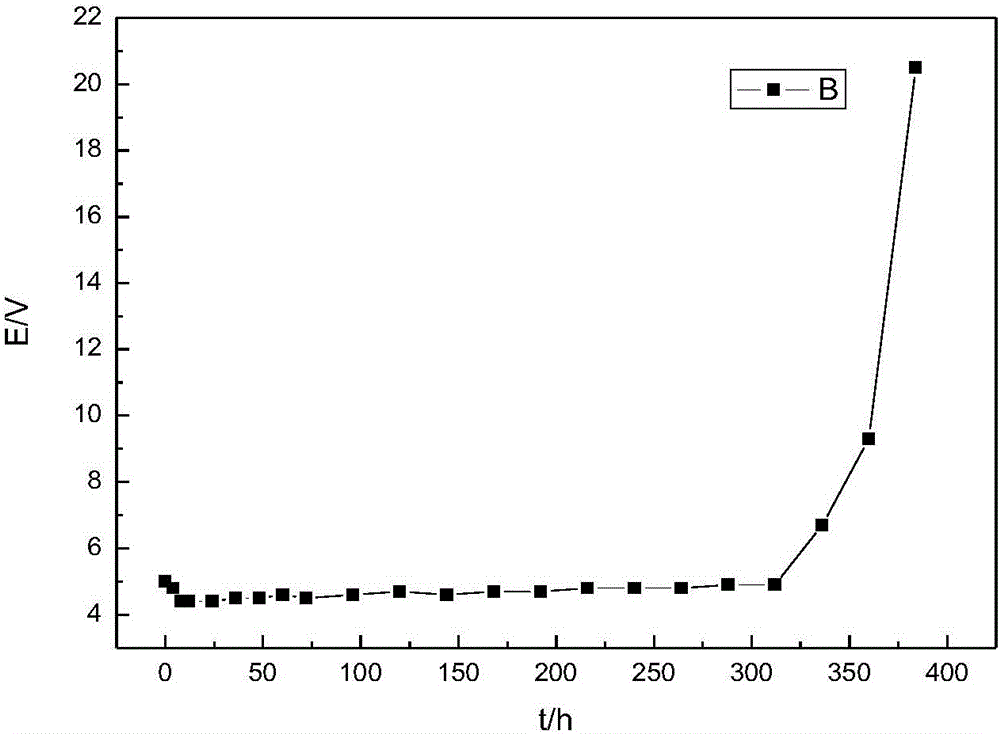
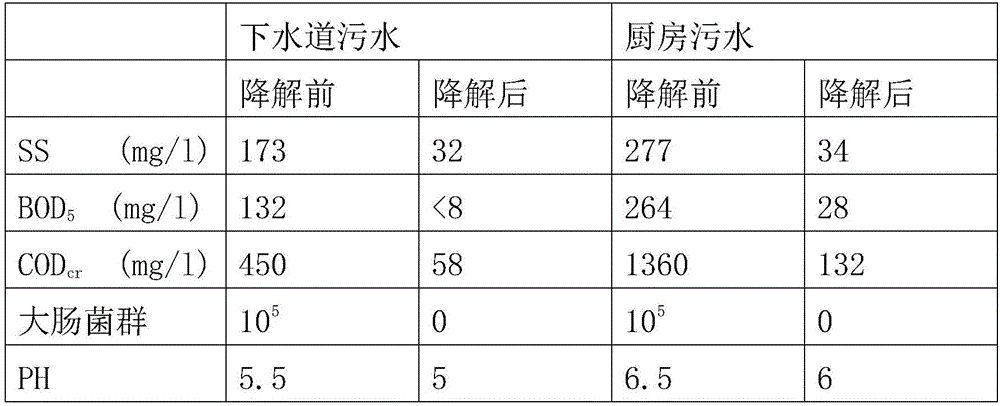
[0002] The new technology of electrolytic
sewage treatment has many advantages such as strong
processing capacity, small equipment size, and no secondary
pollution. It has been widely used to treat wastewater containing organic pollution such as hydrocarbons, alcohols, aldehydes, ethers, and
phenols. The main Depending on the oxidation reaction on the surface of the
anode, the
organic matter is directly oxidized and degraded on the surface of the anode, or the
intermediate product with strong oxidative properties is electrolyzed, and the oxidation of the organic matter and the competition reaction of
oxygen evolution are carried out on the anode. - The wastewater with more ions is mainly the
precipitation of
chlorine gas; in the process of electrolytic treatment of organic wastewater, the electrode not only plays the role of transmitting current, but also catalyzes the oxidation and degradation of organic matter. A necessary condition for the catalytic electrode is to have a
High oxygen evolution (
chlorine evolution)
overpotential, so the choice of
electrode material directly affects the degradation efficiency of organic matter. The
electrode material should have excellent catalytic activity and
conductivity, long
corrosion resistance stability and electrode life, wide Electrochemical
wide mouth, higher current efficiency and lower price, electrode materials in the prior art include
metal electrodes,
graphite electrodes,
platinum electrodes,
metal oxide electrodes and
boron-doped
diamond film electrodes, wherein
metal electrodes are in
Dissolution is prone to occur during the
electrolysis process, which not only consumes metal electrode materials, but also brings new substances into the solution to pollute the solution, and a
passivation film will be formed on the metal surface to affect the
conductivity and activity of the electrode; the catalytic performance of the
inert electrode
platinum electrode The activity is not high, and the
platinum electrode is prone to pollution and inactivation during the
electrolysis process; the
graphite electrode itself has poor strength, and the electrode loss is large when the
current density is high; the
metal oxide electrode is formed on a metal base (such as Ti, Zr, Ta , Nb, etc.) on which a layer of metal oxide film is deposited has the advantages of low loss and stable dimensions during the
electrolysis process: a
mixed metal oxide coated electrode disclosed in
Chinese patent 201520001915.4 and its preparation method and disclosed in 201510646610.3 A Ti / Sb-SnO 2 / Nd-TiO 2 -PbO 2 The preparation of the electrode and the method of degrading active blue 117 involve the preparation methods of several metal oxide anodes. In the complex sewage environment, the
oxygen evolution (
chlorine evolution) potential is too low, and the stability of the
surface chemical composition and properties is not stable. Good,
short life, limited application fields, complex modification work and other issues, not suitable for degrading pollutants such as
phenol;
boron-doped
diamond thin film electrodes have excellent mechanical properties, electrical conductivity and wide
electrochemical potential Window, high sewage degradation efficiency, capable of degrading difficult-to-degrade pollutants containing
phenol, an organic
sewage treatment device using
boron-doped
diamond electrodes disclosed in
Chinese patent 201510289210.1, and P-N junction characteristics BDD-TiO disclosed in 200810103518.2 2
Electrode preparation method and Pd / 3DOMTiO disclosed in 201410186591.6 for photoelectric catalytic
reduction treatment of organic pollutants 2 The preparation method of / BDD electrode and its application relate to the preparation method of boron-doped diamond film electrode and the corresponding electrolysis device. However, due to the high price of boron-doped diamond film electrode, the high cost of industrial production, and the difficulty in preparing large-scale electrodes, etc.
Restrict its
industrial scale production and use;
Chinese patent 201510422402.5 discloses an electrode material with a
titanium oxide intermediate
coating, which involves an electrode material with a
titanium oxide intermediate
coating, using titanium, aluminum and their alloys as electrodes The inner core structure, the surface is coated with
titanium oxide (TiO) with excellent electrochemical properties such as low resistivity,
high activity, and good
electrochemical corrosion resistance. 4 o 7 ), in order to improve the conductivity of the electrode and protect the inner core material, and then prepare a highly active and cheap metal
oxide coating on its surface by
electroplating or use a
thermal decomposition method to prepare a highly active rare metal oxide, which is only used as an intermediate layer In the field of electrolytic
metallurgy; Chinese patent 201410230151.6 discloses a titanium oxide conductive
ceramic electrode preparation process using in-situ
dehydrogenation technology to prepare sintered electrodes, which has the problems of active impurities on the surface and reduced conductivity; Chinese patent 201510716682.0 discloses a
Copper metal plate carrier and titanium oxide film involved in the preparation of
copper-based titanium oxide electrode plate, the main component of titanium oxide film is Ti 4 o 7 and TiO 2 , where Ti 4 o 7 The content of titanium oxide is not less than 50% of the total
mass of the film. During the long-term electrolysis process with
high current, the breakdown potential of the
copper substrate is low, and it is easy to corrode and fail.
Titanium oxide cannot completely cover the electrode surface; Chinese patent 201410166118.1 is disclosed The preparation method of a titanium / titanium oxide / lead
composite substrate is to roughen the surface of the
titanium plate before
electroplating lead, then sinter a layer of titanium oxide, and then use the conductivity of titanium oxide to plate a layer of titanium oxide on its surface. layer metal lead, to obtain a titanium / titanium oxide / lead
composite substrate for
bipolar lead-acid batteries, mainly used in lead-acid batteries, compatible with the substrate material; due to the preparation method process of the above titanium oxide electrode Complicated, the electrode itself has the disadvantages of low conductivity and reduced
overpotential of
oxygen evolution reaction
 Login to View More
Login to View More  Login to View More
Login to View More