Solid medium for cryopreservation of corneal model, preparation method and application method
A solid medium and cryopreservation technology, applied in the field of biomedicine, can solve problems such as tissue viability decline, apoptosis, and tissue structure damage, and achieve the effects of stimulating cell growth and migration, inhibiting cell apoptosis, and inhibiting aging genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
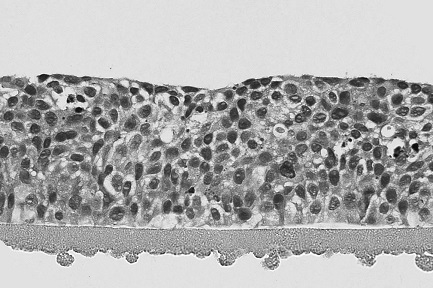
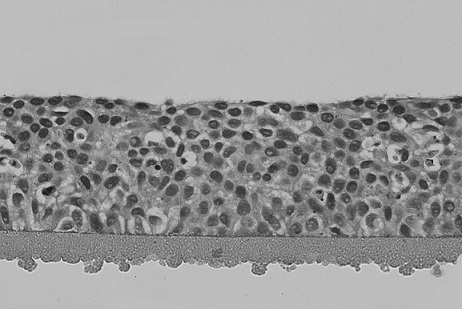
[0026] The solid culture medium for corneal model cryopreservation of the present embodiment is made by mixing the basal medium and the agarose aqueous solution according to the volume ratio of 1:1 and then solidifying; the mass concentration of the agarose aqueous solution is 0.5 %, the agarose is a low-melting point agarose, the gelling temperature of the low-melting point agarose is 28°C, and the melting temperature is 62°C; Bovine serum, retinoic acid, human epidermal growth factor, insulin, hydrocortisone, adenine, triiodothyronine, glutamine and cryoprotectant; DMEM culture in the mixed solution of the DMEM culture medium and F12 culture medium The volume ratio of medium and F12 culture medium is 3:1, the volume percentage content of fetal bovine serum in the described basal medium is 5%, the concentration of retinoic acid is 4 μ g / mL, and the concentration of human epidermal growth factor is 0.1 ng / mL. mL, the concentration of insulin is 5.0ng / mL, the concentration of h...
Embodiment 2
[0035] The corneal model of the present embodiment is a solid medium for cryopreservation, and the solid medium is made by mixing the basal medium and the agarose aqueous solution according to a volume ratio of 1:1 and then solidifying; the mass concentration of the agarose aqueous solution is 1.0 %, the agarose is a low melting point agarose, the gelling temperature of the low melting point agarose is 32°C, and the melting temperature is 68°C; Bovine serum, retinoic acid, human epidermal growth factor, insulin, hydrocortisone, adenine, triiodothyronine, glutamine and cryoprotectant; DMEM culture in the mixed solution of the DMEM culture medium and F12 culture medium The volume ratio of medium and F12 culture medium is 1:1, the volume percentage content of fetal bovine serum in the described basal medium is 20%, the concentration of retinoic acid is 10 μ g / mL, and the concentration of human epidermal growth factor is 1.0 ng / mL. mL, the concentration of insulin is 20.0ng / mL, th...
Embodiment 3
[0044]The corneal model of the present embodiment is a solid medium for cryopreservation, and the solid medium is made by mixing the basal medium and the agarose aqueous solution according to a volume ratio of 1:1 and then solidifying; the mass concentration of the agarose aqueous solution is 5 %, the agarose is low-melting point agarose, the gelling temperature of low-melting point agarose is 30°C, and the melting temperature is 65°C; Bovine serum, retinoic acid, human epidermal growth factor, insulin, hydrocortisone, adenine, triiodothyronine, glutamine and cryoprotectant; DMEM culture in the mixed solution of the DMEM culture medium and F12 culture medium The volume ratio of medium and F12 culture medium is 1:3, the volume percentage content of fetal bovine serum in the described basal medium is 10%, the concentration of retinoic acid is 4 μ g / mL, and the concentration of human epidermal growth factor is 0.5 ng / mL. mL, the concentration of insulin is 10.0ng / mL, the concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com