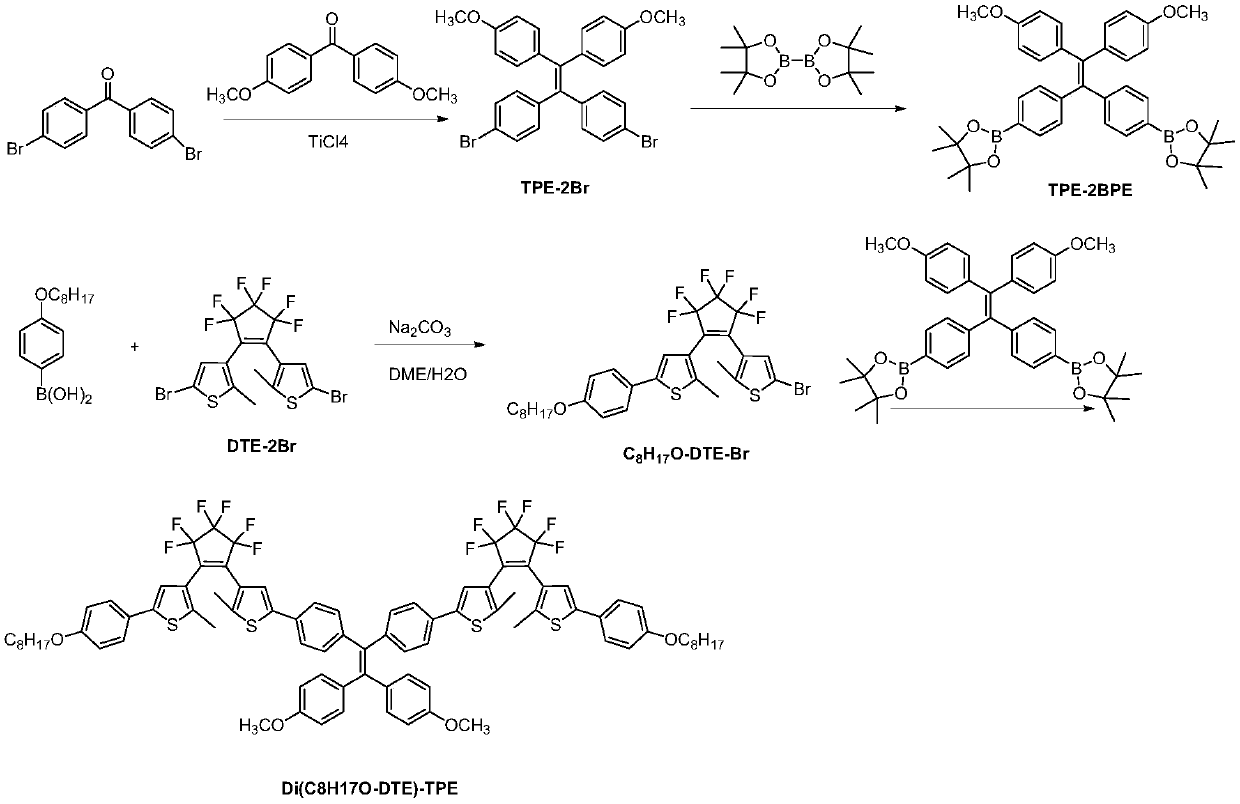
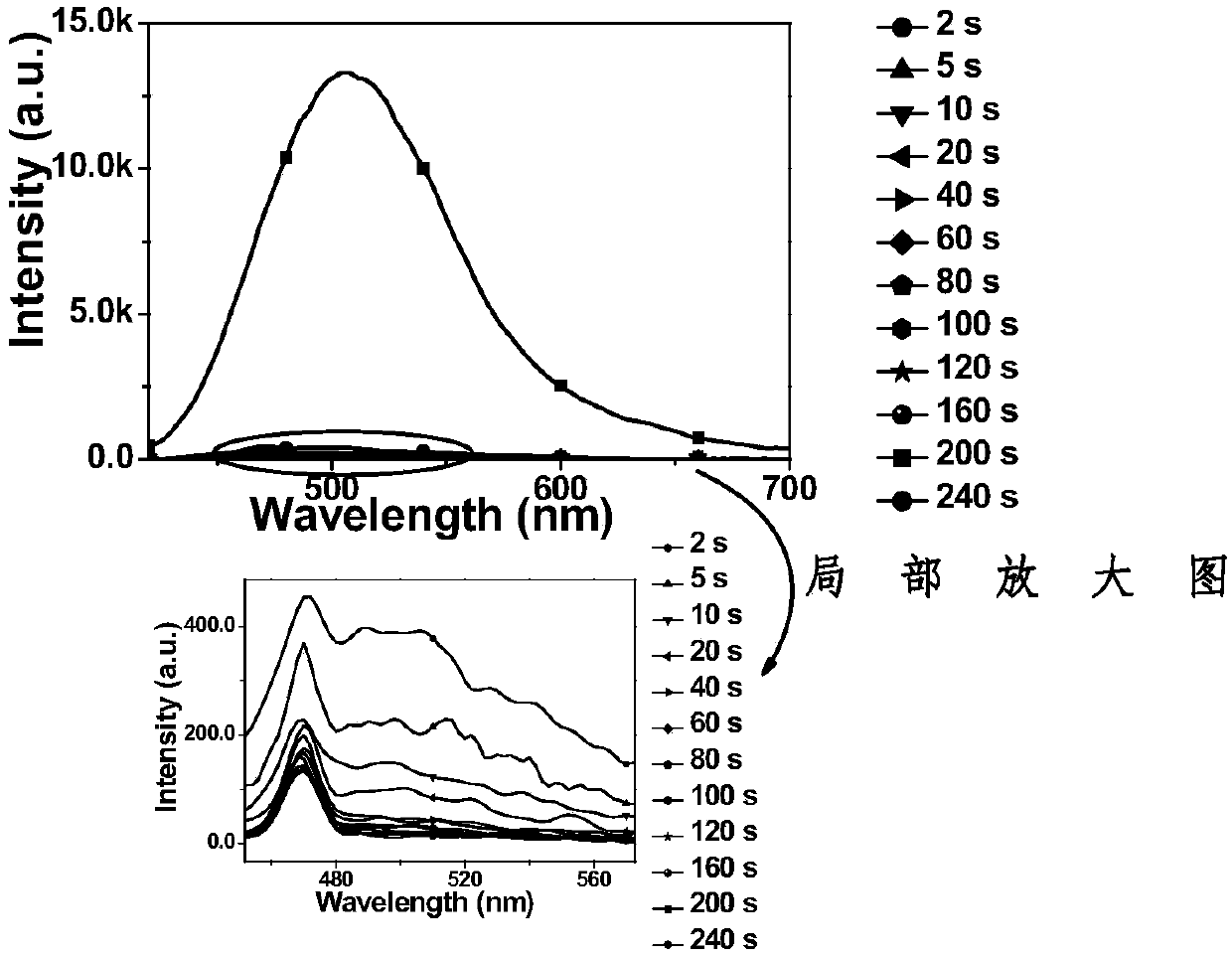
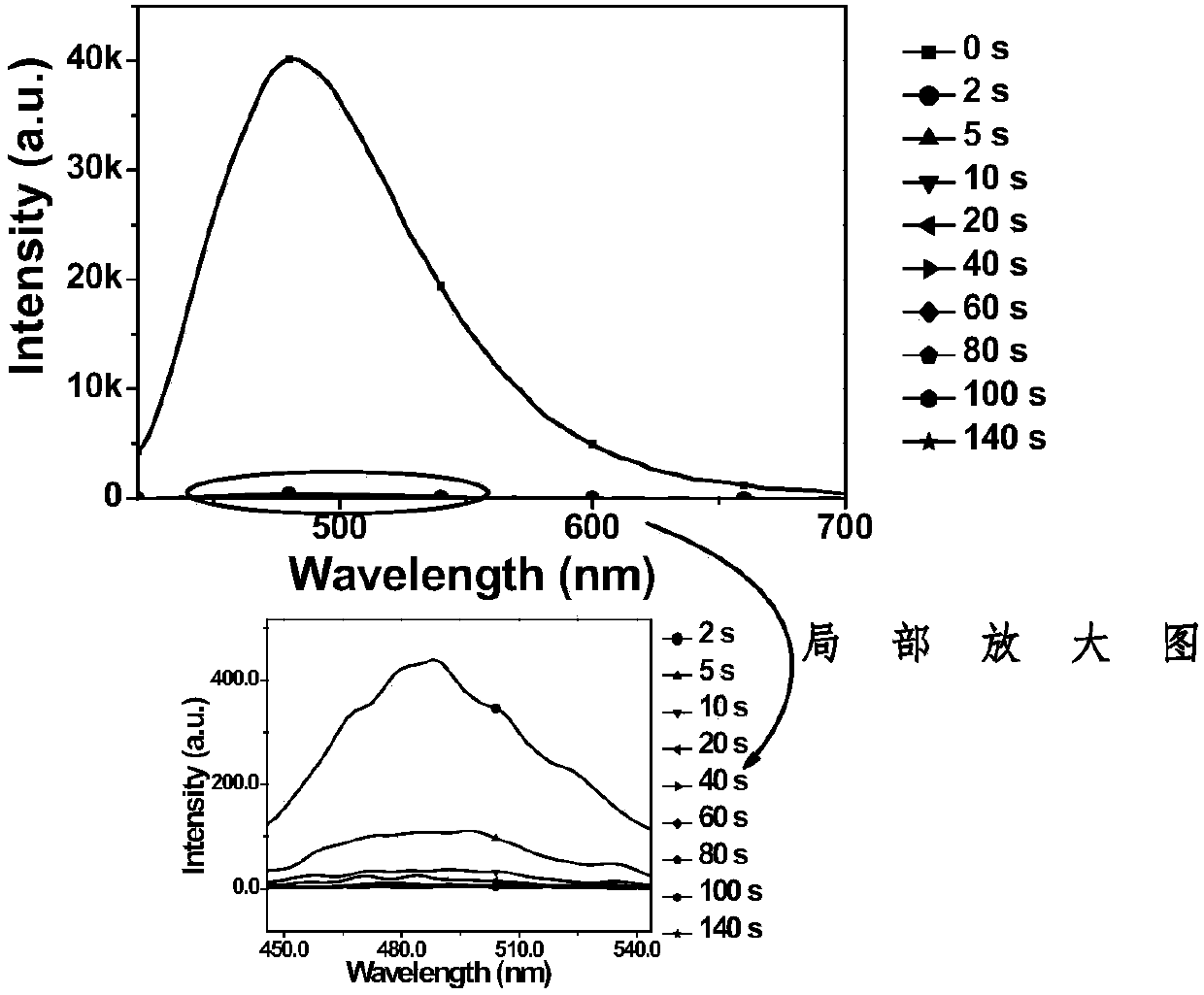
Diarylethene fluorescent molecular switch and preparation method and application thereof
A technology of diarylethene and fluorescent molecules, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, and can solve problems such as low fluorescence quenching efficiency, no visible light response, and high fluorescence switching ratio. , to achieve high quantum yield, rich molecular switch system, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation method of the above fluorescent molecular switch comprises the following steps:
[0060] (1) Utilize Mcmurry reaction to couple intramolecular or intermolecular ketone carbonyls into olefinic bonds, and then Miyaura reaction occurs to generate boronic acid ester or boronic acid by halogen group to obtain tetraarylethene nucleus;
[0061] (2) For an aryl group containing two active sites, use Suzuki reaction to modify the active site at one end of the aryl group to obtain a diarylethene nucleus that retains the active site at the other end;
[0062] (3) Suzuki coupling reaction is performed between the tetraarylethene core obtained in step (1) and the diarylethene core obtained in step (2) which retains the active site at the other end.
[0063] Specifically, in the structure of formula (II), R 1 and R 2 for R 3 When it is a methoxy group, the preparation method of the corresponding fluorescent molecular switch includes the following steps:
[0064]...
Embodiment 1
[0082] A diarylethene fluorescent molecular switch shown in formula (2), whose name is abbreviated as Di(C 8 H 17 O-DTE)-TPE, where R 1 and R 2 for R 3 is methoxy. Its synthetic route is as figure 1 shown, including the following steps:
[0083] (1) ethylene glycol dimethyl ether was pretreated with nitrogen for 30 minutes to remove dissolved oxygen, and the raw materials were p-n-pentoxyphenylboronic acid (0.90 g, 3.6 mmol), 1,2-bis(5-bromo-2methylthiophene) -3-yl) perfluorocyclopentene (DTE-2Br) (1.58g, 3mmol) and sodium carbonate (1.59g, 15mmol) were uniformly dispersed in a mixture of ethylene glycol dimethyl ether and water according to the feeding ratio of 1:1.2:5 In the mixed solution of 4:1, the catalyst tetrakis (triphenylphosphine) palladium (0.17 g, 0.15 mmol) was added under nitrogen atmosphere, and the mixture was pumped 3 to 4 times. The reaction temperature was 90° C. and the reaction time was 20 hours. After completion of the reaction, the organic laye...
Embodiment 2
[0089] A diarylethene fluorescent molecular switch as shown in formula (1), whose name is abbreviated as Di(C 8 H 17 O-DTE)-OTPE, R 1 and R 2 for R 3 is H and X is O. Its synthetic route is as Figure 7 shown, including the following steps:
[0090] (1) Mix zinc powder with anhydrous tetrahydrofuran, slowly inject titanium tetrachloride (2.639 mL, 23.52 mmol) at 0°C under nitrogen atmosphere, reflux for 3 to 5 hours, cool down to room temperature, and add the raw material 2,2'-dichloromethane Bromobenzophenone (2g, 5.88mmol) and Zhanxanthone (1.154g, 5.88mmol) were dissolved in THF at a charging ratio of 1:1 and were added to the system quickly, and the system was pumped for 3 to 4 times. The reaction temperature was 80°C, and the reaction time for 5 hours. Use 10% K after the reaction 2 CO 3 The reaction was quenched and the organic layer was collected and purified by column chromatography to give the product OTPE-2Br.
[0091] (2) Potassium acetate (0.934g, 9.54m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com