Novel ceftriaxone sodium for injection
A technology for ceftriaxone sodium and injection, which is applied in the field of medicine, can solve the problems of staying in the microcapsule technology, not being converted into commercial products, and being few in number, etc., and achieves the effects of shortening the medication interval, low cost, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 prepares p-dioxanone-lactide copolymer of the present invention
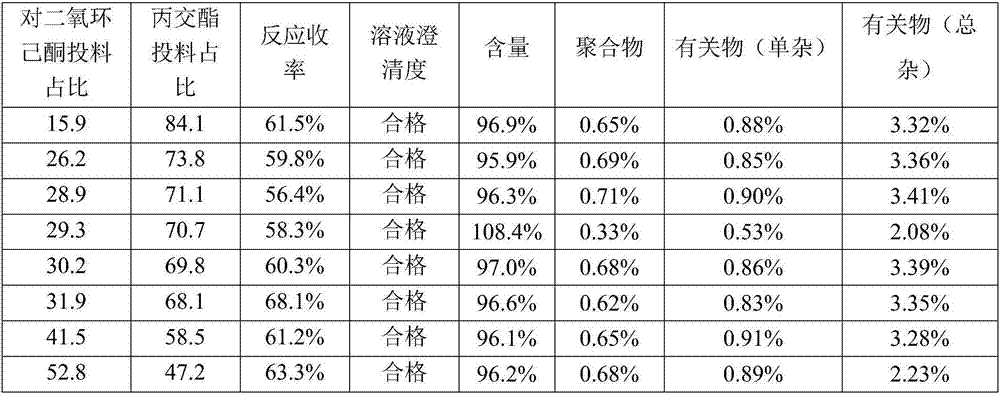
[0022] P-dioxanone and lactide are put into a three-necked flask according to the weight ratio, and catalyst stannous octoate (3.56% of the lactide monomer quality) and initiator lauryl alcohol (lactide) are added under the protection of nitrogen. 2.72% of the monomer mass), vacuumed to 10Pa, heated to 185°C under stirring, reacted for 1.5h, stopped stirring, kept vacuum for 4h, and then naturally cooled to room temperature. Dissolve the product with a small amount of dichloromethane, then settle with petroleum ether 3 times the volume of the solution, and filter with suction, repeating this process 3 times, and finally the obtained copolymer product is dried under reduced pressure at 40°C for 3 hours in a vacuum drying oven to obtain different proportions The finished product of dioxanone-lactide copolymer.
Embodiment 2
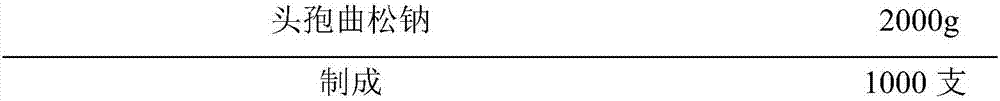
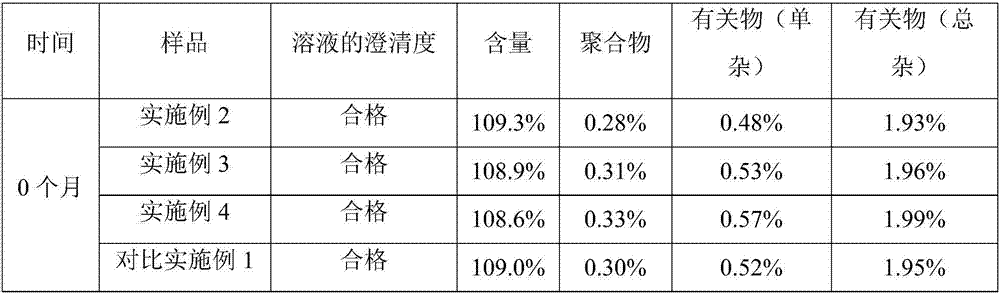
[0023] Embodiment 2 prepares ceftriaxone sodium for injection according to the present invention, specification: 1.0g
[0024] prescription:
[0025] Ceftriaxone Sodium
1000g
333g
20L
p-dioxanone-lactide copolymer (29.3:70.7)
667g
267g
Span-80
889ml
80L
[0026] The specific steps are:
[0027] 1. Firstly crush ethyl cellulose and ceftriaxone sodium respectively, and pass through 100-mesh and 80-mesh sieves. 2. Dissolve the prescribed amount of ethyl cellulose in the prescribed amount of acetone, add the prescribed amount of ceftriaxone sodium and the stabilizer p-dioxanone-lactide copolymer (29.3:70.7), stir for 20 minutes, Suspension A is obtained. 3. Add the prescribed amount of surfactant Span-80 into the prescribed amount of liquid paraffin, heat in a water bath at 35°C and stir for 20 minutes to obtain solution B. 4. Add suspension A...
Embodiment 3
[0028] Embodiment 3 prepares ceftriaxone sodium for injection according to the present invention, specification: 0.5g
[0029] prescription:
[0030] Ceftriaxone Sodium
500g
150g
12L
p-dioxanone-lactide copolymer (29.3:70.7)
300g
talcum powder
150g
Span-80
450ml
liquid paraffin
35L
[0031] The specific steps are:
[0032] 1. Firstly crush ethyl cellulose and ceftriaxone sodium respectively, and pass through 100-mesh and 80-mesh sieves. 2. Dissolve the prescribed amount of ethyl cellulose in the prescribed amount of acetone, add the prescribed amount of ceftriaxone sodium and the stabilizer p-dioxanone-lactide copolymer (29.3:70.7), stir for 20 minutes, Suspension A is obtained. 3. Add the prescribed amount of surfactant Span-80 into the prescribed amount of liquid paraffin, heat in a water bath at 35°C and stir for 20 minutes to obtain solution B. 4. Add suspension A to so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com