Conjugated polymer material capable of being processed by green solvent and preparation method of conjugated polymer material
A technology of conjugated polymers and green solvents, applied in the field of conjugated polymer materials, can solve the problems of restricting commercial green production, and achieve the effects of wide absorption spectrum, good thermal stability, and obvious color change range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] The preparation method of the above-mentioned green solvent-processable conjugated polymer material comprises the following steps:
[0085] Under the interaction between the catalyst and the ligand, the structural monomers of formula (II-1), formula (II-2), formula (II-3) and formula (III) are subjected to Stille coupling reaction to obtain formula (I ) Conjugated polymers containing oligoethylene glycol side chain structure with structural repeating units, the reaction temperature is 110°C-250°C, and the reaction time is 10-48 hours;
[0086]
[0087]
[0088]
[0089] The structural formula of Q is as follows:
[0090]
[0091] In the formula, m is an integer of 2-10; Z is Cl, Br or I; X is S or Se; E is F or Cl.
[0092] The molar ratio of the structural monomers of formula (II-1), formula (II-2), formula (II-3) and formula (III) is 1:(0.8-1.5), preferably, the molar ratio is 1: 1.
[0093] The catalyst is a palladium catalyst, specifically one or more...
Embodiment 1
[0104] Synthesis of monomer
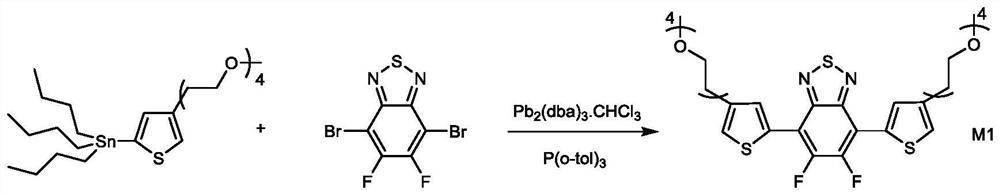
[0105] Synthesis of M1
[0106] Under the protection of argon, the monomer 4,7-dibromo-5,6-difluoro-2,1,3-benzothiadiazole (1mmol, 0.33g) and tris(dibenzylideneacetone Dipalladium)-chloroform adduct (0.08mmol, 83mg), tri(o-methylphenyl)phosphine (0.64mmol, 195mg), 15ml of anhydrous toluene were added to the reaction flask, followed by addition of tin monomer (2.4mmol , 1.35g), the temperature was raised to 110°C, and after 24 hours of reaction, the antipyretic was stopped. After removing the organic solvent by rotary evaporator. The crude product was purified by silica gel column chromatography using methanol / ethyl acetate (1:100) to obtain 0.2 g of orange-yellow liquid, yield 27.8%
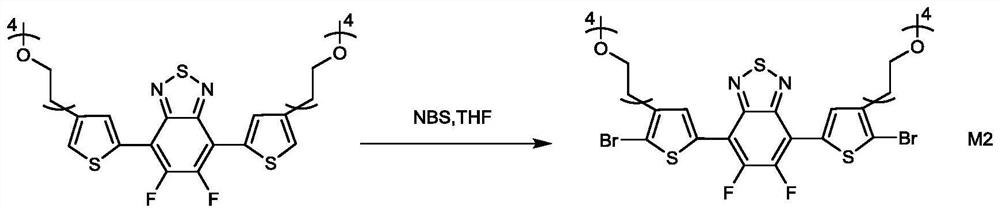
[0107] Synthesis of M2
[0108]Add 8ml of anhydrous tetrahydrofuran into the reaction bottle, and then add NBS (2.4mmol, 0.43g) to disperse NBS in tetrahydrofuran, then add monomer M1 into the reaction system, and react at room temperature for 24 hours. After th...
Embodiment 2
[0110] Polymer Synthesis
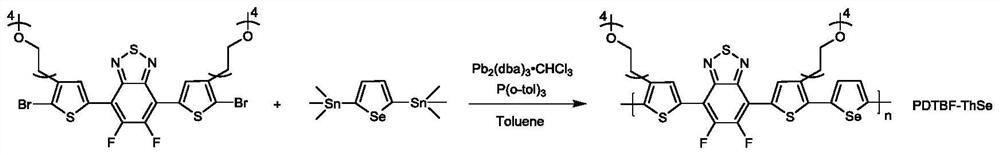
[0111] 1. Synthesis of PDTBF-ThSe
[0112] Under the condition of argon protection, monomer 2,5-bistrimethyltin-selenophene (0.2mmol, 91.3mg), M4 (02mmol, 174.9mg), catalyst tris(dibenzylideneacetone dipalladium)- Chloroform adduct (6mmol, 6.2mg), ligand tri(o-methylphenyl)phosphine (0.048mmol, 14.6mg) were added in the reaction flask, then injected 3ml of anhydrous toluene into the reaction system, and the temperature was raised to 110°C, react for 48 hours. After the reaction, the polymer was sequentially extracted in petroleum ether, methanol, ethanol, ethyl acetate and chloroform to finally obtain a reddish-brown polymer with metallic luster.
[0113] 2. Synthesis of PDTBF-Th
[0114] Under the condition of argon protection, monomer 2,5-bistrimethyltin-based thiophene (0.2mmol, 82.0mg), M2 (0.2mmol, 174.9mg), catalyst tris(dibenzylideneacetone dipalladium)- Chloroform adduct (6mmol, 6.2mg), ligand tri(o-methylphenyl)phosphine (0.048mmol, 14.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com