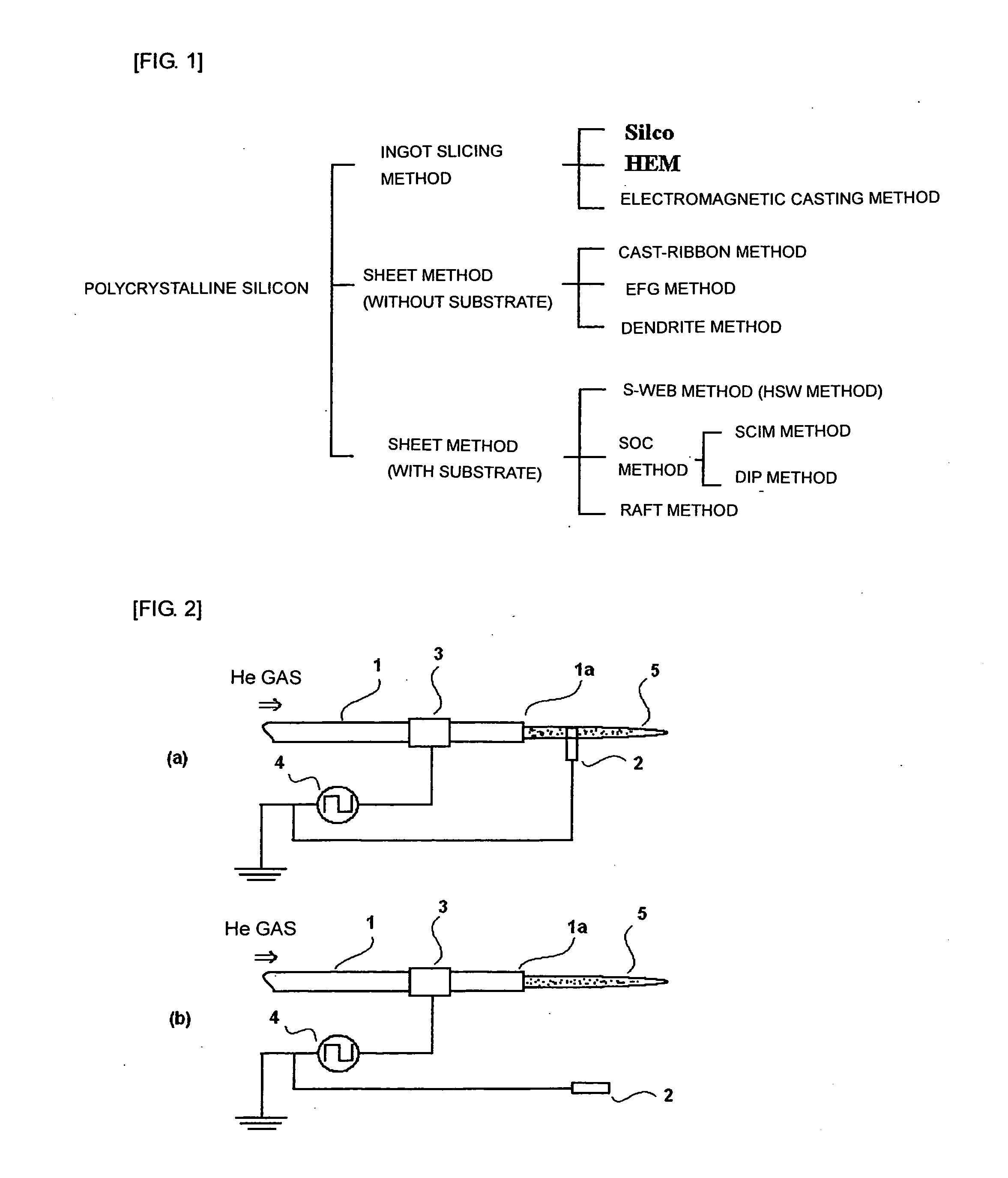
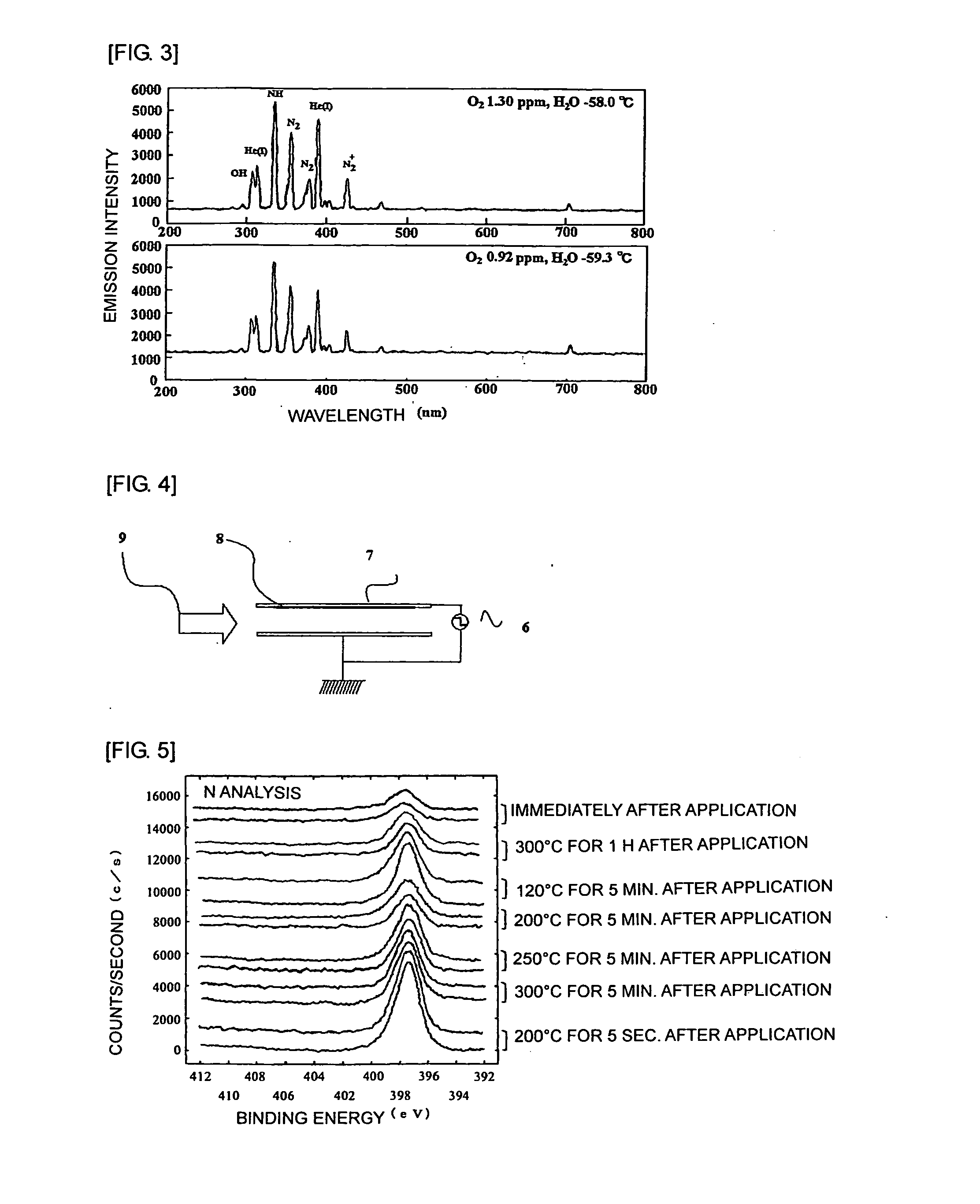
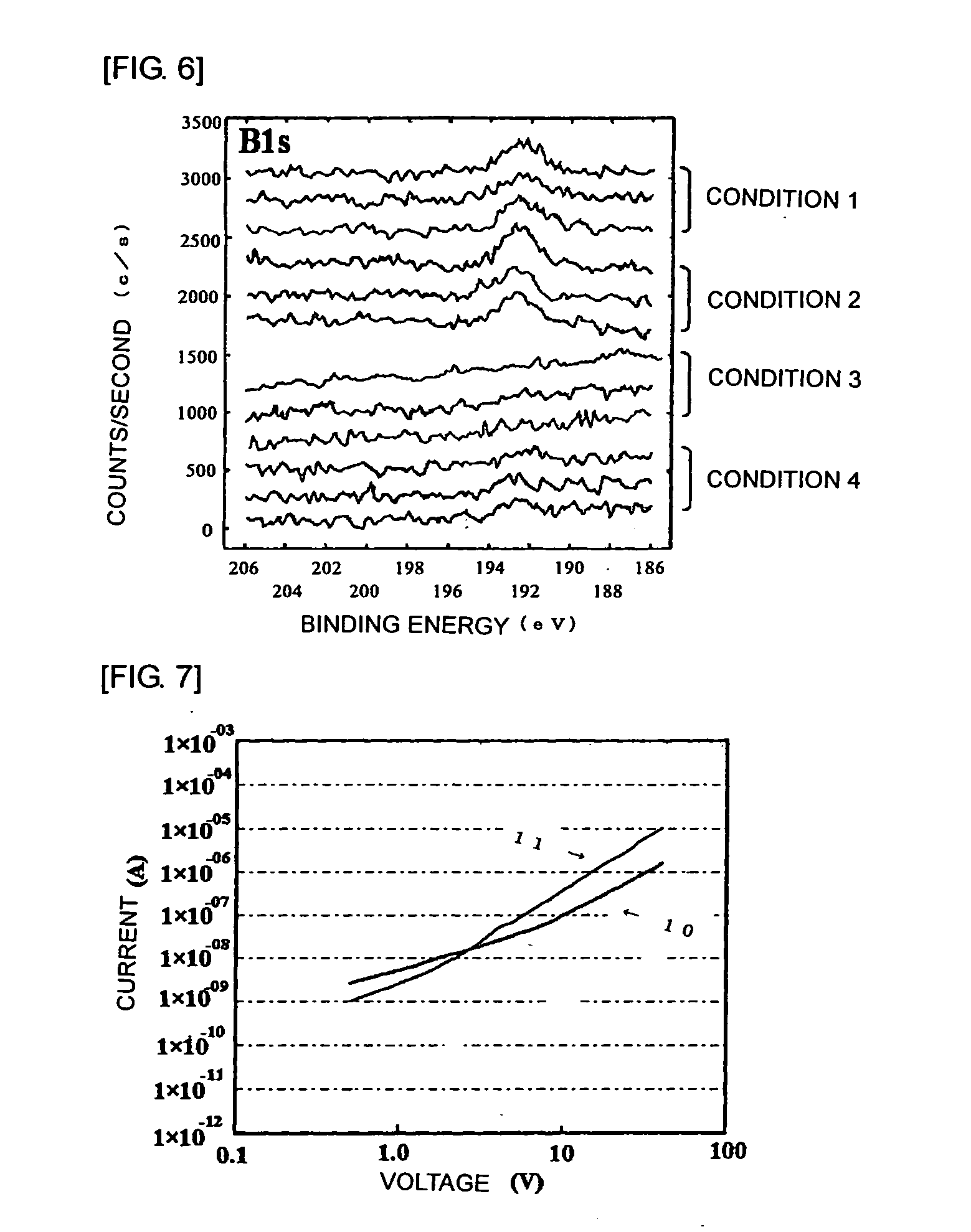
Method for forming bond between different elements
a technology of metal bonding and metal parts, applied in the direction of nitrogen oxide/oxyacid, sustainable manufacturing/processing, final product manufacturing, etc., can solve the problems of unstable transistor characteristics, low mobility disadvantage, and inability to stabilize the characteristics of such semiconductors at all, so as to achieve high energy, high energy component, and high conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of Catalyst, Diphenyl Dicyclopentadienyl Zirconium Cp2ZrPh2
[0113]In a nitrogen atmosphere, into a 300-mL reaction flask, dichloro dicyclopentadienyl zirconium Cp2ZrCl2 (5.0 g) and 39 mL of DME (1,2-dimethoxyethane) as a solvent were charged, and the temperature of the mixture was set to 0 to 10° C. To the mixture, 34.37 mL of solution of phenylmagnesium bromide (PhMgBr) in THF (tetrahydrofuran) having a concentration of 37.1 mol / L was added dropwise at the same temperature, and the whole was stirred at 24 to 26° C. for 19 hours. The reaction mixture was concentrated under reduced pressure at 20° C. / 20 Torr, then 8 mL of diethyl ether (Et2O) was added, and the whole was stirred at 24 to 26° C. for 1 hour. To the resultant mixture, 39 mL of toluene was further added, then the whole was stirred at the same temperature for 30 minutes, and the reaction solution was filtered. The filtrate was concentrated under reduced pressure at 20° C. / 10 Torr, and the obtained solid was wash...
synthesis example 2
Synthesis of Polyphenylsilane
[0114]In a nitrogen atmosphere, into a 100-mL reaction flask, diphenyl dicyclopentadienyl zirconium Cp2ZrPh2 (0.165 g) synthesized in Synthesis Example 1 was charged as a catalyst, then phenylsilane PhSiH3 (10 g) was added to the catalyst at 24 to 26° C., and the mixture was stirred at the same temperature for 89 hours. To the resultant mixture, toluene (47 g) was added, then 3% HCl (68 g×5 times) was added, and the whole was stirred and washed. The organic phase was separated, then ion-exchanged water (68 g) was added, and the whole was stirred and washed. The organic phase was purified by Florisil (27 g) column chromatography using toluene (118 g) as an eluant followed by concentration, and the product was dried at 80° C. for 2 hours to yield polyphenylsilane (8.87 g) as a target compound. The obtained polyphenylsilane was composed of a chain polyphenylsilane corresponding to Formula (1) and a cyclic polyphenylsilane corresponding to Formula (2) in a m...
synthesis example 3
Synthesis of Polyhydrosilane
[0115]To a 100-mL brown reaction flask, polyphenylsilane (5.0 g) synthesized in Synthesis Example 2 and cyclohexane (43.5 g) as a solvent were charged. To the mixture, aluminum chloride AlCl3 (0.41 g) was added, and the whole was solidified with liquid nitrogen. The temperature of the mixture was raised to room temperature in a water bath followed by nitrogen substitution. Into the mixture, hydrochloric acid HCl gas was blown at a flow rate of 950 mL / min for 10 hours. Then, decompression and pressure return by nitrogen were repeated 10 times to remove hydrochloric acid HCl. To the mixture, 13.72 g of solution containing lithium aluminum hydride LiAlH4 (1.17 g) in diethyl ether (Et2O) was added dropwise at 0 to 10° C. over 30 minutes in a nitrogen atmosphere. The whole was stirred at room temperature for 12 hours, and the reaction solution was poured into ion-exchanged water (11 g). The mixture was stirred for 1 minute and left. Then, the supernatant liqui...
PUM
| Property | Measurement | Unit |
|---|---|---|
| output voltage | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com