However, the energy content of lead acid batteries is rather low.
A long recharge time, for example, of about 2 hours required for lead acid batteries necessitates in many applications, a cumbersome mechanical swap of a discharged battery by a charged battery.
Although
nickel-
metal hydride batteries provided better performance than the lead acid batteries, for example, a
driving range of about 60 km, a
specific energy of about 60 Wh / Kg to about 90 Wh / Kg, an
energy density of about 200 Wh / L-300 Wh / L, a specific power of about 200 W / kg, and an electric recharge of about 3 hours, albeit at a higher cost of about $1,000 / kWh, the
nickel-
metal hydride batteries were not an acceptable replacement for
gasoline from the customer's perspective.
Despite the dedicated work of many scientists and engineers worldwide, the
hydrogen fuelled
polymer electrolyte membrane fuel
cell (PEMFC) technology did not result in a market success of electric vehicles.
The reasons are as follows: to achieve practically useful
power density on the positive electrode, high
platinum (Pt) loading is required which increases the cost of the PEMFCs; the
dissolution of a Pt catalyst at positive potentials makes the positive electrode less durable; the lack of an inexpensive, sustainable, and a clean
hydrogen source; and the lack of a
hydrogen manufacturing and distribution infrastructure.
However, the first
lithium batteries had a poor cycle life since the electronically insulating
surface film formed on
metallic lithium leads to dendritic Li plating during recharge.
However, fully electric vehicles, unlike plug-in hybrids, based on
lithium ion batteries (LIBs) did not achieve a widespread commercial success, primarily due to a low energy content, that is, a low
driving range, and a high
total cost of ownership of the batteries.
The often quoted statistics that 60% of daily car trips in the United States are less than 60 Km is apparently not helping the sales of
lithium-
ion battery powered cars as most drivers need the capability to make longer trips.
Apart from the low
driving range, the LIBs also have a low electric recharge rate, for example, the Nissan Leaf® takes about 30 minutes for a charge of about 80% of full capacity, and the construction of a large scale battery swapping infrastructure is not justified due to the lack of a sizable LIB
electric vehicle market, as illustrated by a recent bankruptcy of Better Place.
The scientists at
General Motors (GM) arrived at the same conclusion, that is, the battery electric vehicles based on current and targeted Li
ion battery technology will be limited to small vehicle, low mileage-per-day applications due to relatively low
specific energy and long recharge time constraints, and it is possible that fundamental
physical limitations may prevent pure Li ion based battery electric vehicles (BEVs) from delivering the freedom of providing long trips, with intermittent quick refills, that consumers currently receive from their cars.
We're going to have a more significant breakthrough and probably go into some other area of battery
chemistry.” MIT's Yet-Ming Chiang concurs: “It is clear that long-term vehicle
electrification—especially affordable 200
mile all-electric range—will require batteries with approximately three times greater energy densities at about one third the cost per kWh than that of LIBs.” Kevin See, analyst for Boston-based
Lux Research, said “It is not realistic or feasible for automakers to significantly
cut the price of lithium-ion batteries.
There is going to be incremental improvement, but we don't believe it will be enough to spur the huge adjustment everyone was hoping for.” Tesla Motors has conceded that new technologies will eventually be required.
It is just too expensive and they're too heavy.”
According to Takeshi Uchiyamada, Toyota's Vice Chairman, “the current capabilities of electric vehicles”, based on
fuel cells or lithium ion batteries, “do not meet society's needs, whether it may be the distance the cars can run, or the costs, or the long time to charge.
Because of its shortcomings, that is, driving range, cost, and recharging time, the battery or fuel
cell electric vehicle is not a viable replacement for most conventional cars.
Conventional
redox flow batteries such as
vanadium redox flow batteries have a low
energy density that translates into a short driving range, because the components have low solubilities and a large amount of an otherwise useless
solvent which has to be carried on-board to keep the components in the fluid state.
Improvements in packing factor, that is the ratio of practical to theoretical
energy density, by using, for example, binder free SEAM batteries with a soluble
mediator or a soluble
redox couple or
metal containing
ionic liquid flow batteries or protected Li
metal anode, run into the fundamental limitation that the intrinsic energy densities of known battery chemistries are not sufficiently high for fully
electric vehicle applications.
Also, the cost of such batteries is likely to stay above the mid-term target of about $100 / kWh and about $30 / kW, or about $2,250 / car with about 100
horsepower.
However, the fundamental problems related to the slow
kinetics of the
oxygen electrode result in high cost and poor durability of PEM
fuel cells due to the necessity of high Pt loading in the case of near ambient temperature
fuel cells.
Another problem with fuel cells, in general, is the source of the fuel, for example, hydrogen.
Paul Zigouras, Director of New
Business Development at EPC Corporation, eloquently summarizes the status quo as: “Flow batteries are a great idea, but unfortunately, no fluid currently exists that will hold a decent amount of energy.
I am hopeful, but also doubtful that a fluid will ever be developed that can effectively do this”.
As a result, F2 has poor
cycle efficiency, in addition to material compatibility issues, whereas I2 has a low energy density in addition to
solubility problems.
However, the chorine cells use an expensive
ruthenium (Ru)-containing catalyst and provide poor energy efficiency.
Although hydrogen-
oxoacid flow batteries such as H2—HNO3 have been considered in the past, these flow batteries have poor
discharge efficiency and lack the ability of electrical recharge or regeneration of the reagents.
However, such reactions did not find applications in
energy storage and conversion, mostly due to their poor reversibility.
Although the use of a
mediator leads in theory to reduced energy efficiency compared to a direct electrode reaction, this thermodynamic loss of energy efficiency is often smaller than the kinetic loss associated with electrode over-
voltage at the same power using oxidants such as
oxygen or using direct electroreduction of the oxoanions.
However, this process irreversibly consumes Ba(OH)2, H2SO4 and generates BaSO4 waste.
Also, this process does not co-produce a stoichiometric amount of hydrogen, which is required for the complete
energy cycle of discharge and regeneration.
Although this method is chemical and waste free, this method has poor energy efficiency and a low
throughput.
For example,
semiconductor based photovoltaic solar panels, for example,
polycrystalline silicon photovoltaic solar panels, multilayer photovoltaic solar panels, InxGa (1−x) Se2, etc., are either inefficient or too expensive.
Photoelectrochemical
water splitting into hydrogen (H2) and
oxygen (O2) using
anatase TiO2 nanoparticles also suffers from a low efficiency due to the high over
voltage of the oxygen production centers.
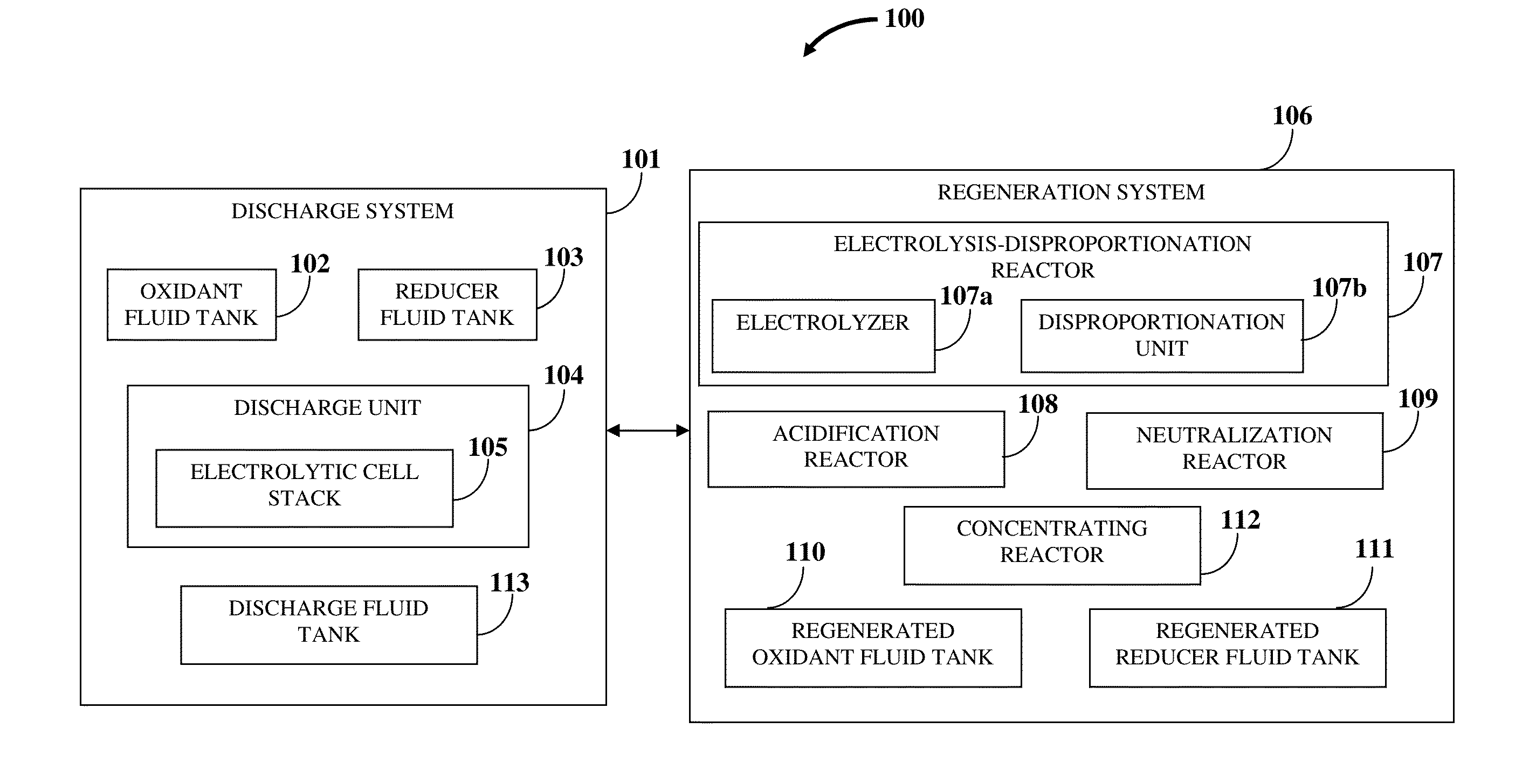
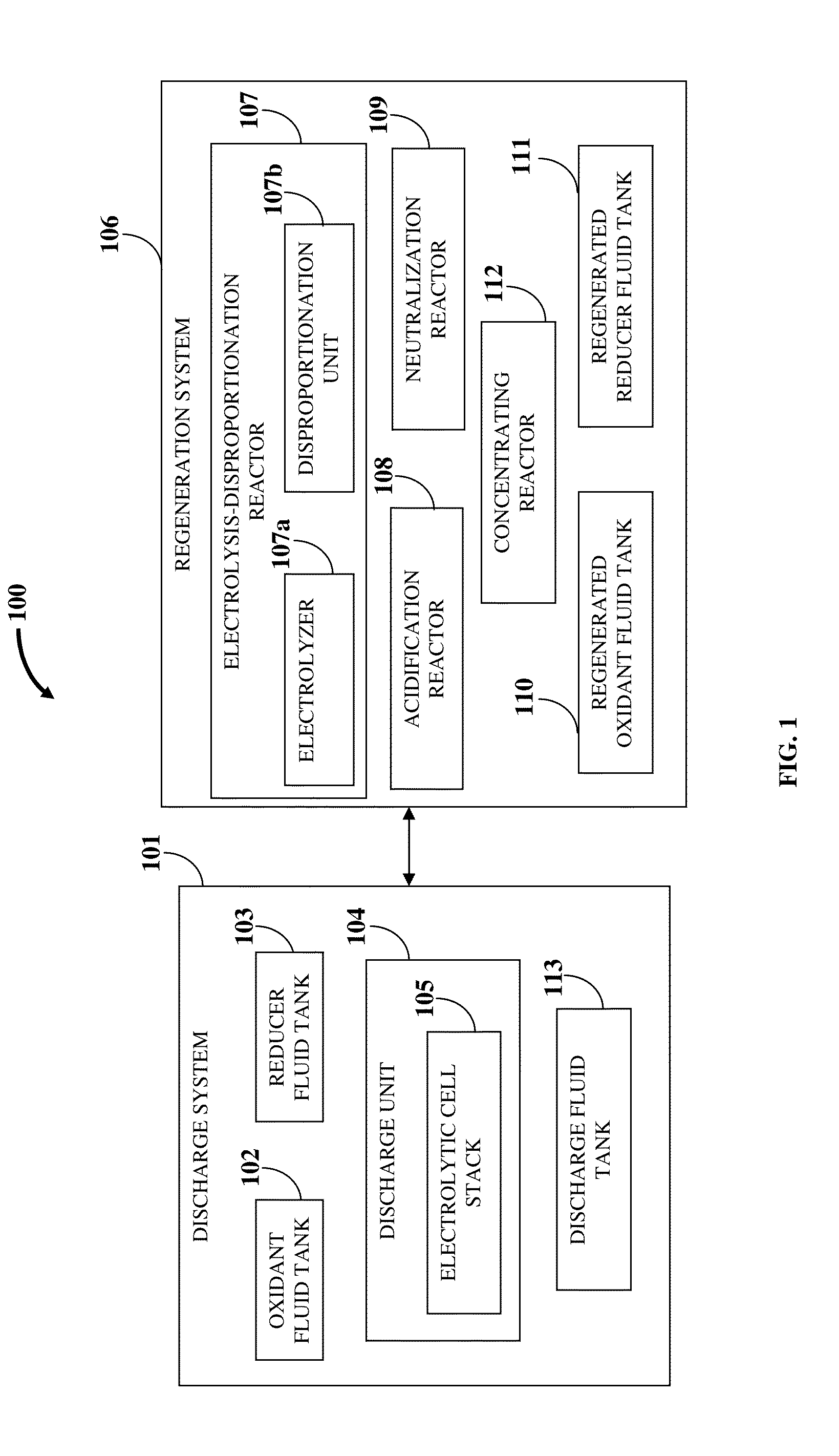
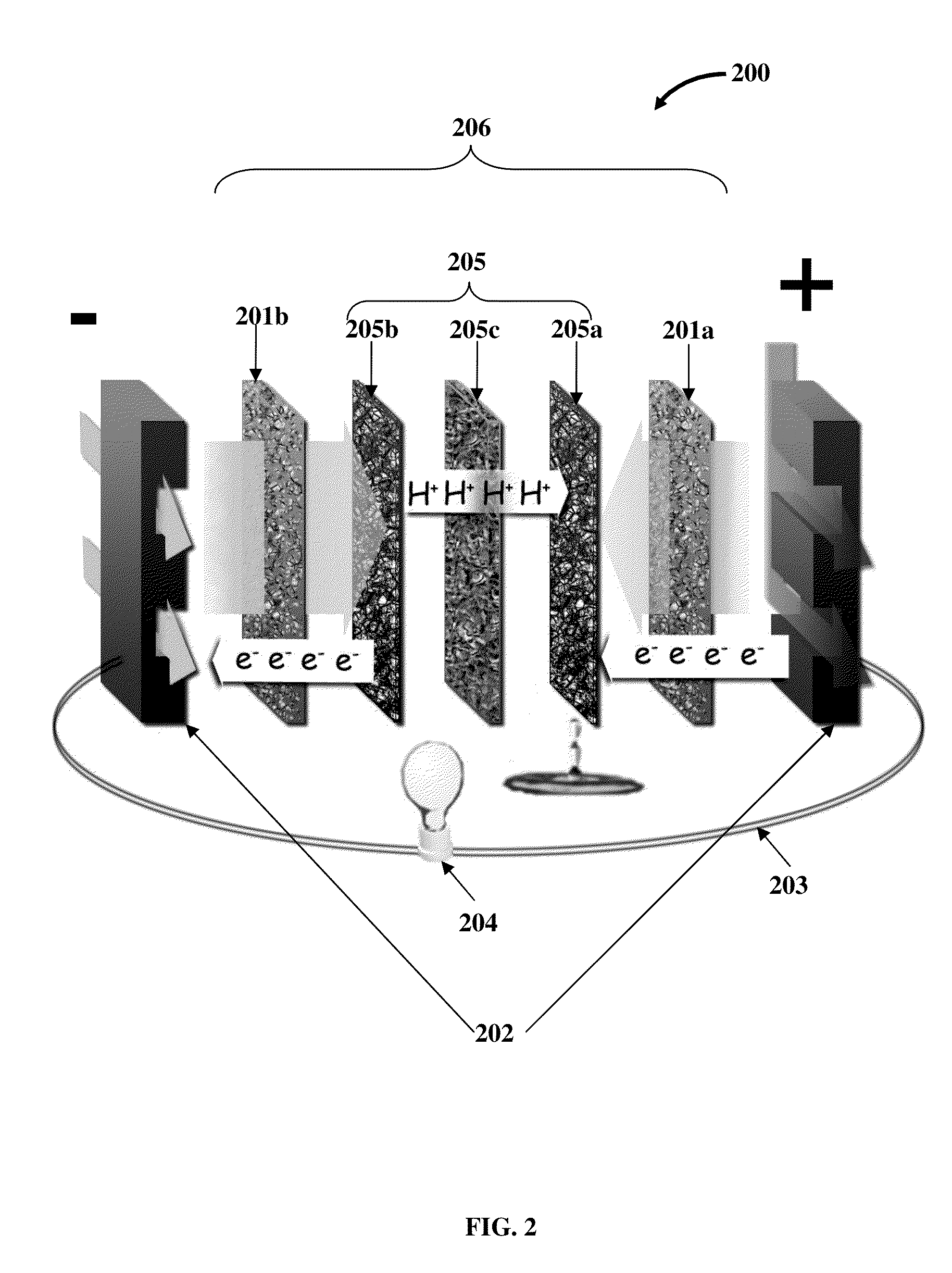
Hence, there is a long felt but unresolved need for an electrochemical
flow battery that provides for a
high energy density, that is, a long driving range, a
high energy efficiency and power at a low operational and manufacturing cost, and requires a short refill time.
 Login to View More
Login to View More