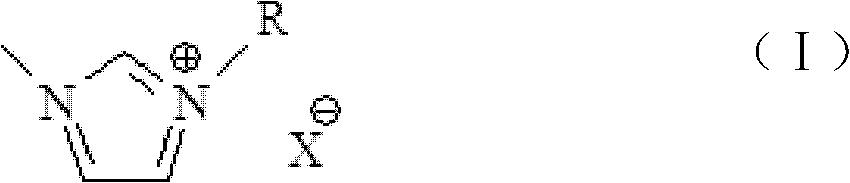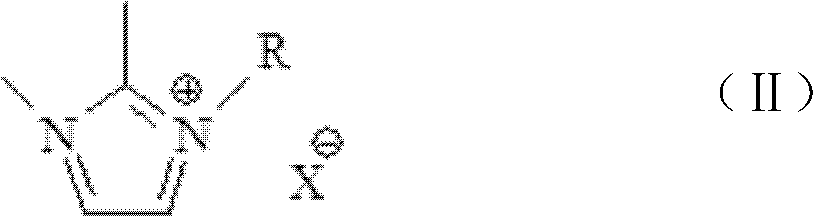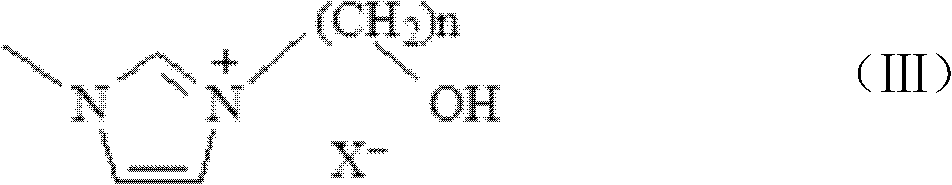Method for synthesizing allantoin
A synthesis method and technology for allantoin, applied in the direction of organic chemistry, etc., can solve the problems of difficult control, high cost, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] (1) Preparation of ionic liquid:
[0110] Put 850g of 1,4-butane sultone in a 2L three-necked flask, and gradually add 410g of N-methylimidazole dropwise at 25°C. The dropwise addition is completed within 30 minutes, and the reaction solution is heated to 40°C, stirred for 12 hours, and filtered to obtain The white solid was placed in 1.6L of toluene and stirred for 15 minutes, filtered with suction, and the obtained crude product was placed in a vacuum drying oven (90°C, dried at 0.01Mpa to constant weight); 919g of the intermediate was obtained. Take 584g of the white powdery solid obtained above, put it into a 2L three-neck flask, add 1.1L of methanol to dissolve, slowly add 265g of sulfuric acid (mass fraction 98%) dropwise, keep the temperature at 80°C after the dropwise addition, and react for 6h. After the reaction finished, the excess moisture was evaporated by the reaction solution with a rotary evaporator, and the obtained [(CH 2 ) 4 SO 3 HMIM][HSO 4 ], wh...
Embodiment 2
[0114] After drying the ionic liquid collected in the reaction of Example 1, add 2.0 L of methanol, and heat to reflux for 2 hours. After the reflux is completed, cool and filter to obtain the filtrate that is concentrated and separate the methanol (it can be used for the next time) to obtain the ionic liquid that can be reused once. Liquid A1, add glyoxylic acid aqueous solution and urea, the molar ratio of glyoxylic acid to urea is 1:4.2, heat the reaction solution to 68°C, react for 6.5h, stop heating, cool to 15°C, filter to obtain a white solid, Wash the solid with 1L of deionized water, wash three times (1L each time), combine the washing liquid and filtrate, concentrate and remove the water to obtain the ionic liquid for repeated use, and dry it in a vacuum drying oven for later use. The white solid obtained by the reaction is the product Allantoin was taken out and dried in an oven at 100°C, weighed, and the calculated yield was 75.06%, which was marked as the second ba...
Embodiment 3
[0118] (1) Preparation of ionic liquid:
[0119] Put 850g of 1,4-butane sultone in a 2L three-necked flask, gradually add 395g of pyridine dropwise at 25°C, and the dropwise addition is completed within 30min, heat the reaction solution to 40°C, stir for 12h, and filter to obtain a white solid. It was placed in 1.6L toluene and stirred for 15min, filtered with suction, and the obtained crude product was placed in a vacuum drying oven (90°C, dried at 0.01Mpa to constant weight) to obtain 896g of intermediate; take the white powder obtained above Add 578g of solids into a 2L three-neck flask, add 1.1L of deionized water to dissolve, slowly add 265g of sulfuric acid (mass fraction 98%) dropwise, keep the temperature at 80°C after the dropwise addition, and react for 6h. The excess water was evaporated with a rotary evaporator to obtain [(CH 2 ) 4 SO 3 HPy][HSO 4 ], it was placed in a vacuum drying oven (90°C, 0.01Mpa, dried to constant weight), and 650g was obtained, which wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com