Erythromycin enteric-coated tablets and preparation method thereof
A technology for erythromycin and enteric-coated tablets, applied in the field of antibiotic drugs and their preparation, can solve the problems of affecting product quality and curative effect, unable to guarantee product qualification, unstable release, etc., and achieve stable drug release effect and preparation process. Ease of control and improved medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
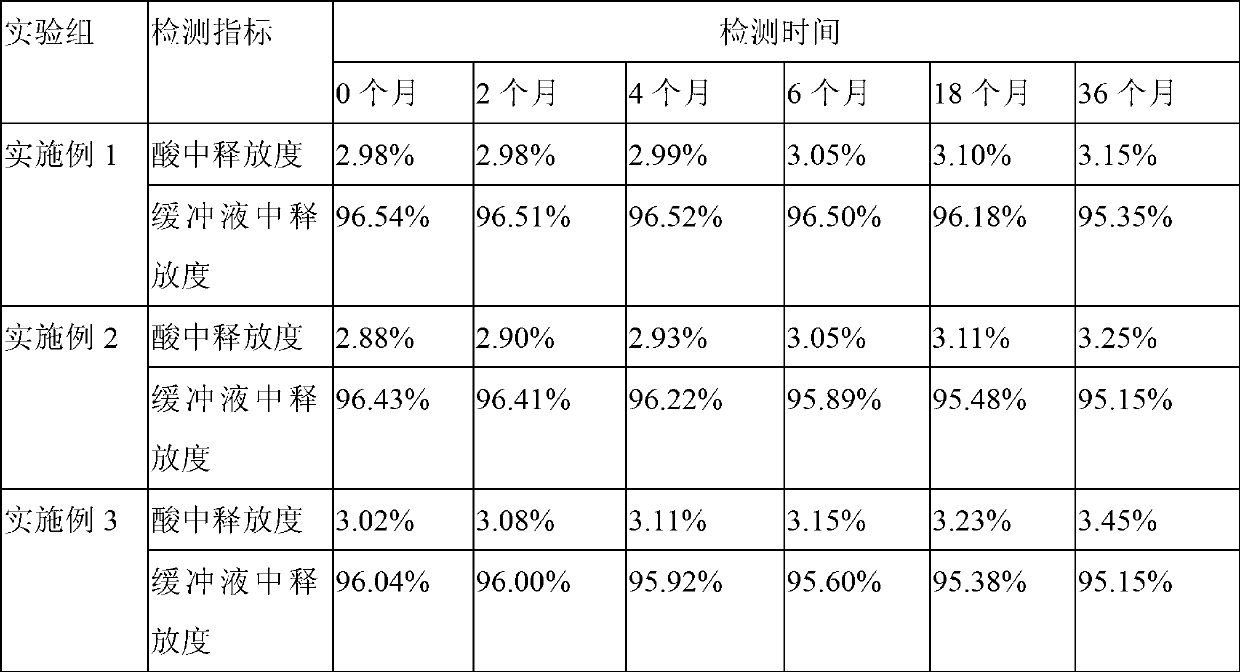
Embodiment 1
[0033] An erythromycin enteric-coated tablet, based on the preparation of 100,000 erythromycin enteric-coated tablets, the core component of the erythromycin enteric-coated tablet is 12.5 billion units of erythromycin (1 billion units / kg), corn Starch 3.4kg, tapioca starch 0.8kg, hypromellose 0.16kg, hypromellose 0.7kg, carboxymethyl starch sodium 0.7kg, magnesium stearate 0.18kg;
[0034] Enteric coating components: polyacrylic resin II 1.0kg, ethanol 13.1kg, diethyl phthalate 0.21kg, castor oil 0.42kg, polysorbate (80) 0.21kg;
[0035] Sugar coating components: 5.8 kg of sucrose, 5.8 kg of talc, 2.0 g of gelatin, 28 g of Sichuan wax, and 2.8 g of simethicone.
[0036] The preparation method is as follows:
[0037] 1. Preparation of tablet core
[0038] (1) Prepare the adhesive, dilute the tapioca starch into a suspension with cold purified water, and stir the hypromellose quickly with hot purified water, and add the hypromellose solution to the suspension In the process, ...
Embodiment 2
[0059] An erythromycin enteric-coated tablet, based on the preparation of 100,000 erythromycin enteric-coated tablets, the core components of the erythromycin enteric-coated tablet are 12 billion units of erythromycin (1 billion units / kg), corn Starch 3kg, tapioca starch 0.5kg, hypromellose 0.1kg, hypromellose 0.5kg, carboxymethyl starch sodium 0.5kg, magnesium stearate 0.1kg;
[0060] Enteric coating components: polyacrylic resin II 0.85kg, ethanol 12kg, diethyl phthalate 0.18kg, castor oil 0.3kg, polysorbate (80) 0.18kg;
[0061] Sugar coating components: 4.5 kg of sucrose, 4.5 kg of talc, 1.0 g of gelatin, 20 g of Sichuan wax, and 1.0 g of simethicone.
[0062] The preparation method is as follows:
[0063] 1. Preparation of tablet core
[0064] (1) Prepare the adhesive, dilute the tapioca starch into a suspension with cold purified water, and stir the hypromellose quickly with hot purified water, and add the hypromellose solution to the suspension In the process, 5kg of...
Embodiment 3
[0078] An erythromycin enteric-coated tablet, based on the preparation of 100,000 erythromycin enteric-coated tablets, the core components of the erythromycin enteric-coated tablet are 13.7 billion units of erythromycin (1 billion units / kg), corn Starch 3.8kg, tapioca starch 1.0kg, hypromellose 0.3kg, hypromellose 1.0kg, sodium starch glycolate 1.0kg, magnesium stearate 0.3kg;
[0079] Enteric coating components: polyacrylic acid resin II 1.15kg, ethanol 15kg, diethyl phthalate 0.24kg, castor oil 0.5kg, polysorbate (80) 0.24kg;
[0080] Sugar coating components: 7.0 kg of sucrose, 7.0 kg of talcum powder, 5.0 g of gelatin, 50 g of Sichuan wax, and 5.0 g of simethicone.
[0081] The preparation method is as follows:
[0082] 1. Preparation of tablet core
[0083] (1) Prepare the adhesive, dilute the tapioca starch into a suspension with cold purified water, and stir the hypromellose quickly with hot purified water, and add the hypromellose solution to the suspension 10kg of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com