Gypenoside lipidosome consisting of sterol and preparation method thereof
A technology of gypenoside and blue saponin, which is applied in the field of pharmaceutical preparations, can solve problems such as no gypenoside liposome reports, and achieve the effects of improving the body's immunity, making it simple, and improving sleep quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] craft
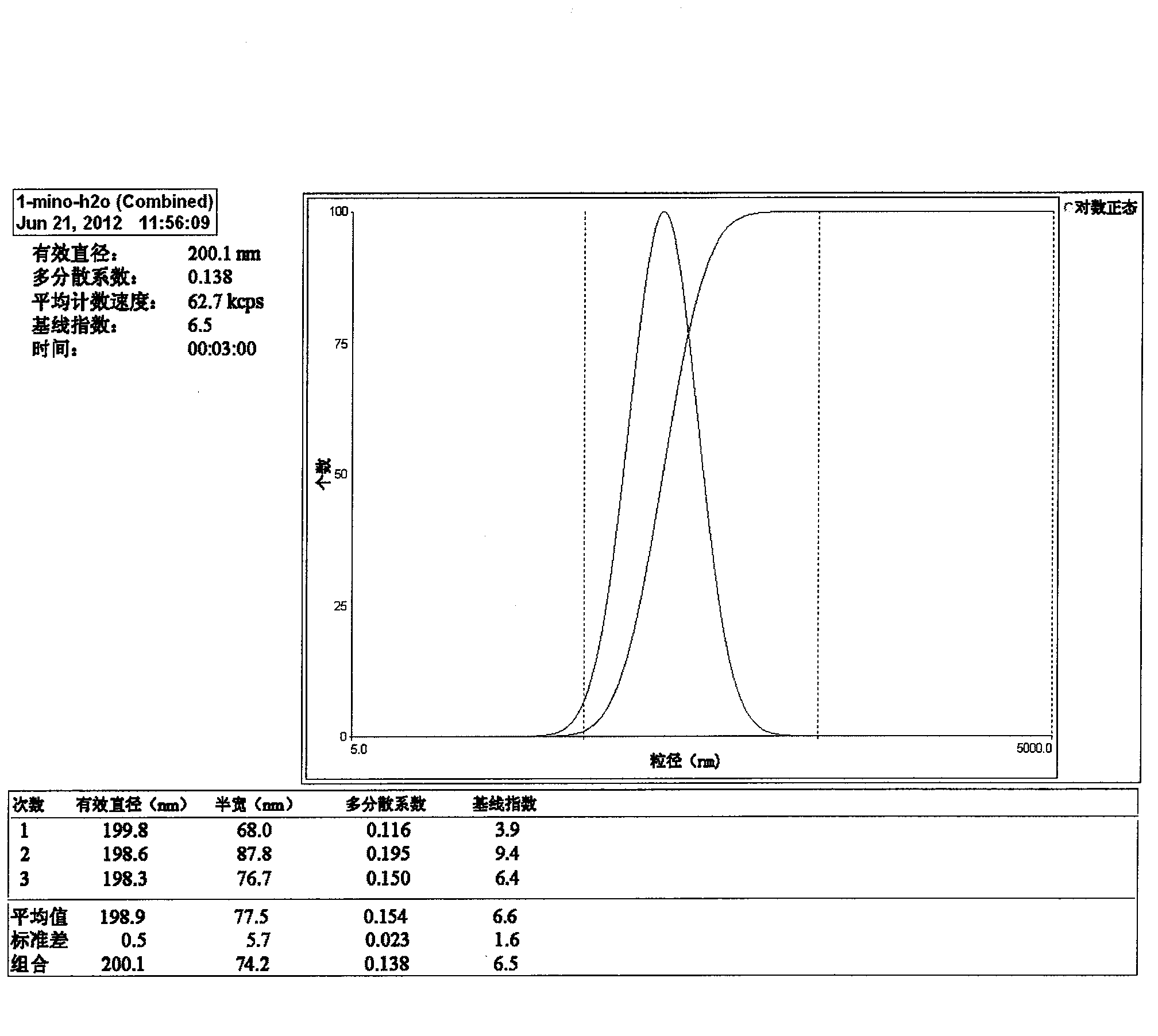
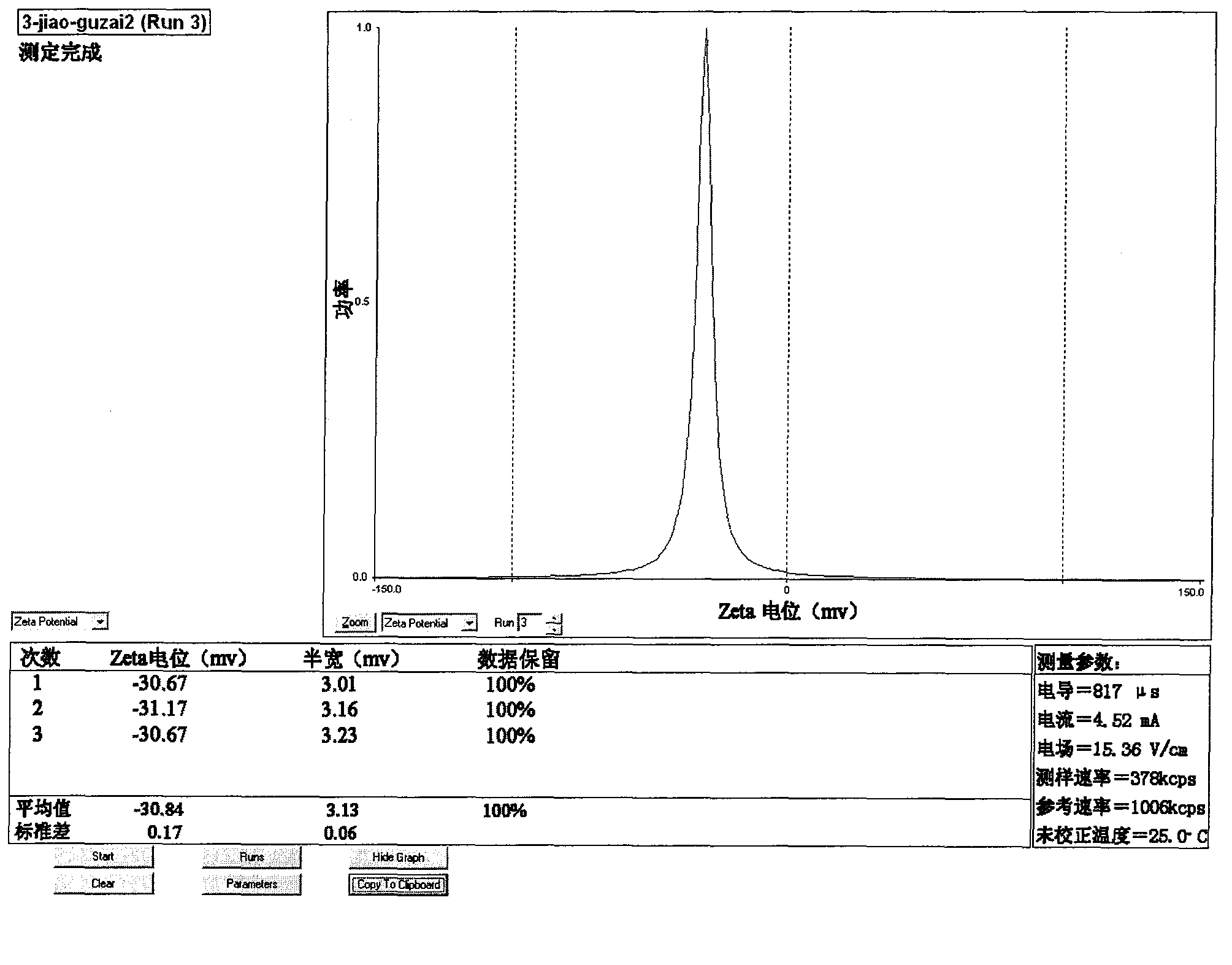
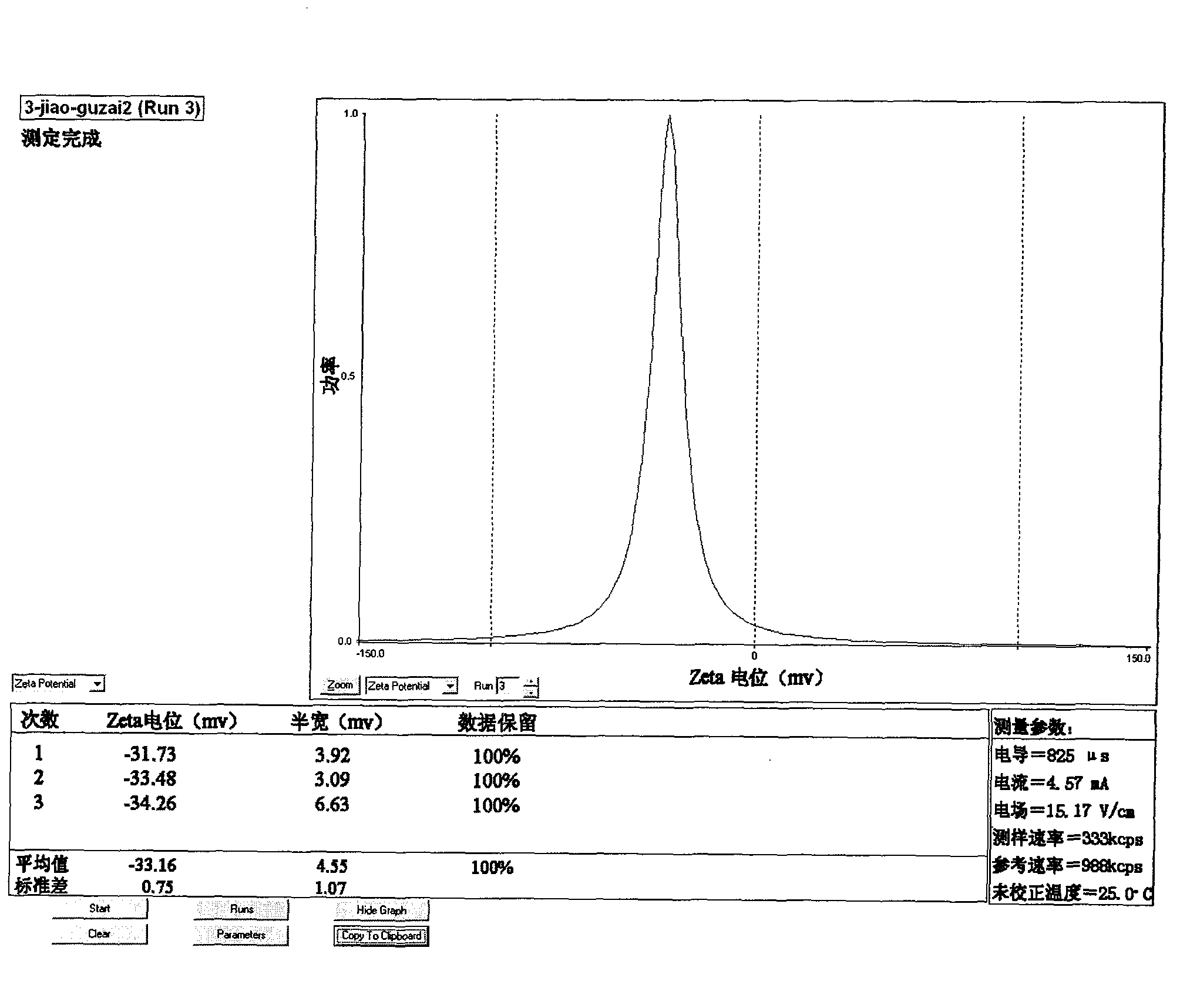
[0043] (1) selection of raw materials: get phosphate buffer saline and gypenoside by above-mentioned prescription, gypenoside is dissolved in phosphate buffer saline, for subsequent use;
[0044] (2) Preparation of gypenoside liposomes: get sphingomyelin, β-sitosterol and ether according to the above prescription, mix well, and then rotary evaporate to make it into a uniform lipid film covering the bottom of the pear-shaped bottle, add Add glass beads to the phosphate buffer solution containing gypenoside, rotate and shake at 60°C for 30 minutes, then place it for 2 hours to fully hydrate.
Embodiment 2
[0047] craft
[0048] (1) Preparation of oil phase: get gypenoside, lecithin, stigmasterol, ethanol according to the above prescription, mix well, as oil phase;
[0049] (2) Preparation of the water phase: take phosphate buffer as the water phase according to the above prescription;
[0050] (3) Preparation of liposomes: under constant temperature stirring, inject the oil phase into the water phase at a temperature of 45°C at a constant speed with a needle, stir at constant temperature for 2 hours to remove ethanol, and obtain the liposome.
Embodiment 3
[0053] craft
[0054] (1) Get phosphatidylserine according to the above prescription: campesterol: dichloromethane=200mg: 50mg: 50mL to prepare the oil phase, place the oil phase in a round-bottomed flask, and spin off the dichloromethane;
[0055] (2) 200mg gypenoside was dissolved in 20mL phosphate buffer (pH=8.0) as the water phase;
[0056] (3) Add 20 mL of phosphate buffer (pH=8.0) containing 200 mg of gypenoside to the oil phase, and in an ice bath, sonicate the probe for 10 minutes to form a uniform emulsion;
[0057] (4) Rotary evaporation to remove the organic phase, after forming a colloidal state, continue to add 20 mL of phosphate buffer, continue to evaporate under reduced pressure for 0.5 h, and ultrasonically probe for 10 min to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com