Acinetobacter baumannii and subunit protein combined vaccine and preparation method thereof
A technology of Acinetobacter baumannii and protein vaccines, which is applied in the interdisciplinary fields of molecular biology and immunology, can solve the problems of small batch-to-batch differences, toxic and side effects, and single ingredients, and achieve fewer adverse reactions, lower incidence rates, and better preparation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Recombinant plasmid construction
[0048] (1) Design and synthesize SmpA and PLD sequence primers according to the SmpA and PLD gene sequences, introduce NdeI and XhoI restriction sites at the two ends of the primers, and synthesize SmpA and PLD genes by PCR;
[0049] (2) The PCR product was recovered and purified, TA cloned into the vector pMD18-T (purchased from Shanghai Haoran Biotechnology Co., Ltd.), and the cloning vector pMD18-SmpA and pMD18-PLD were constructed and transformed into E. coli BL21. The PCR screening was positive For transformants, a small amount of plasmids were extracted, and then identified and sequenced by restriction enzyme digestion;
[0050] (3) The correct pMD18-SmpA and pMD18-PLD were digested and sequenced with NdeI and XhoI, and the pET28b plasmid (purchased from Shanghai Beinuo Biotechnology Co., Ltd.) was ligated with T4DNA ligase to construct the expression plasmid pET28b. -SmpA and pET28b-PLD, and transform them into the expressi...
Embodiment 2
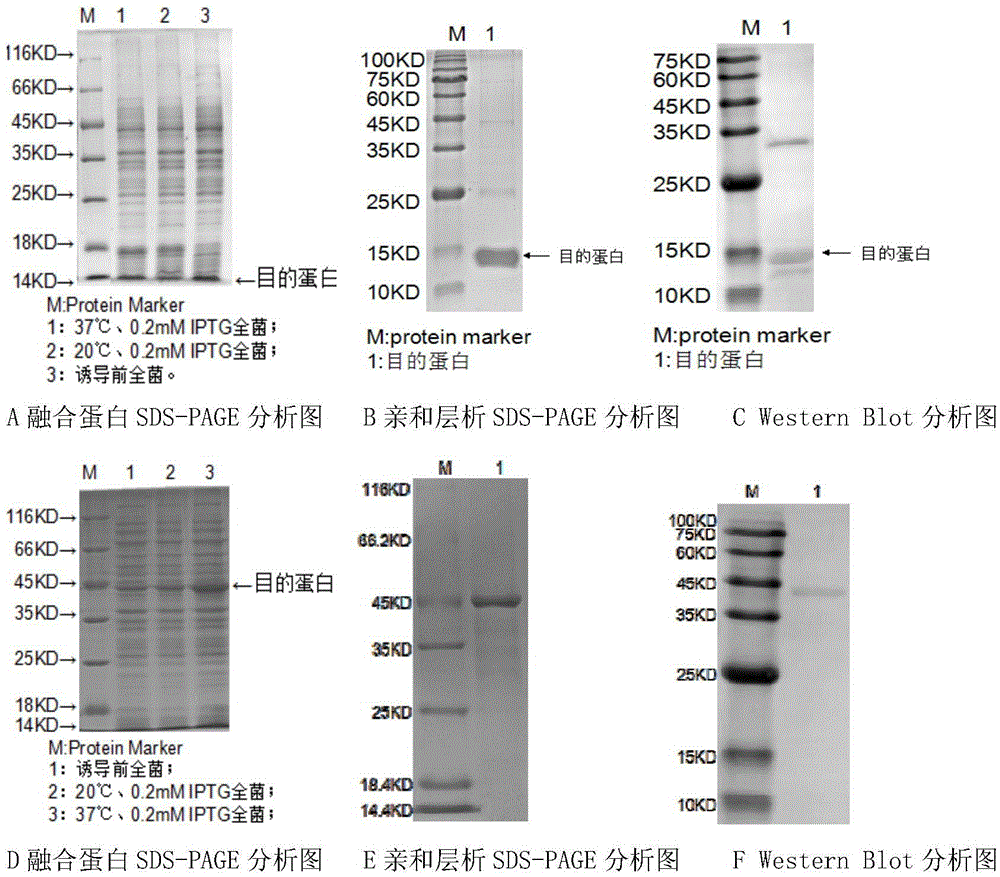
[0051] Example 2: Prokaryotic expression and purification of fusion protein ( figure 1 )
[0052] (1) Take 1ul of the recombinant pET28b plasmid to transform BL21(DE3), heat shock at 42°C for 90s, then stand on ice for 2min, then spread the plate (30ug / mL kanamycin), and incubate at 37°C overnight;
[0053] (2) Pick a single colony of the expression strain BL21(DE3) in a test tube (4mL LB medium, 30ug / mL kanamycin) 37°C, 220rpm overnight culture;
[0054] (3) Inoculate the cultured bacteria liquid in 4ml LB medium at a volume ratio of 1:100, add 30ug / mL kanamycin, and cultivate at 37°C and 220rpm;
[0055] (4) When the OD value reaches about 0.6, add IPTG with a final concentration of 0.2 mM, 20°C overnight, 37°C, 220 rpm for 5 hours, and no IPTG inducer is added as a negative control;
[0056] (5) Collect the bacterial cells and suspend them in PBS buffer;
[0057] (6) SDS-PAGE electrophoresis detection ( figure 1 A, D);
[0058] (7) Mass induction: Inoculate the bacterial solution cultu...
Embodiment 3
[0068] Example 3: SmpA and PLD are respectively immunized in animals to select appropriate doses
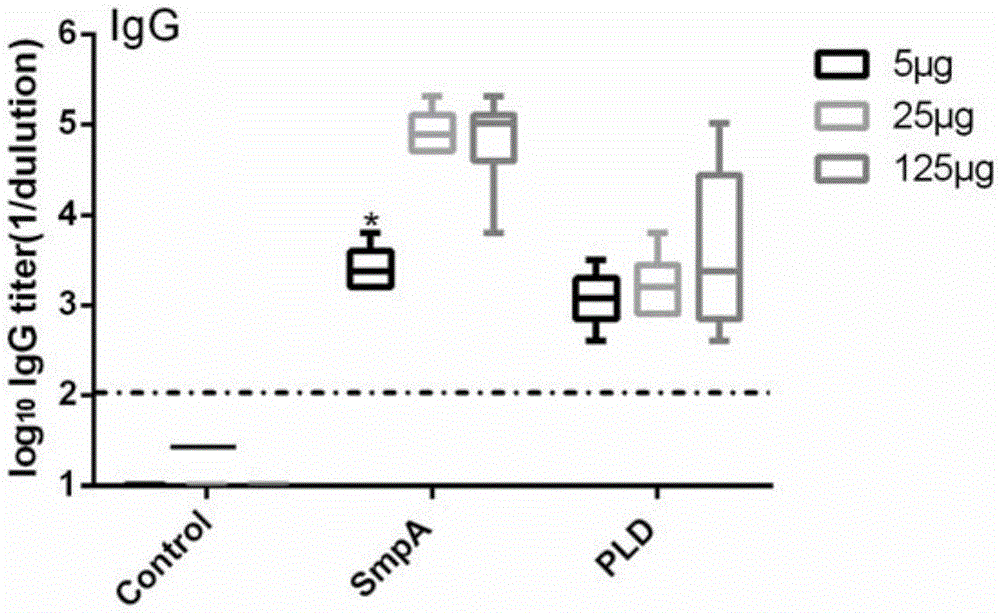
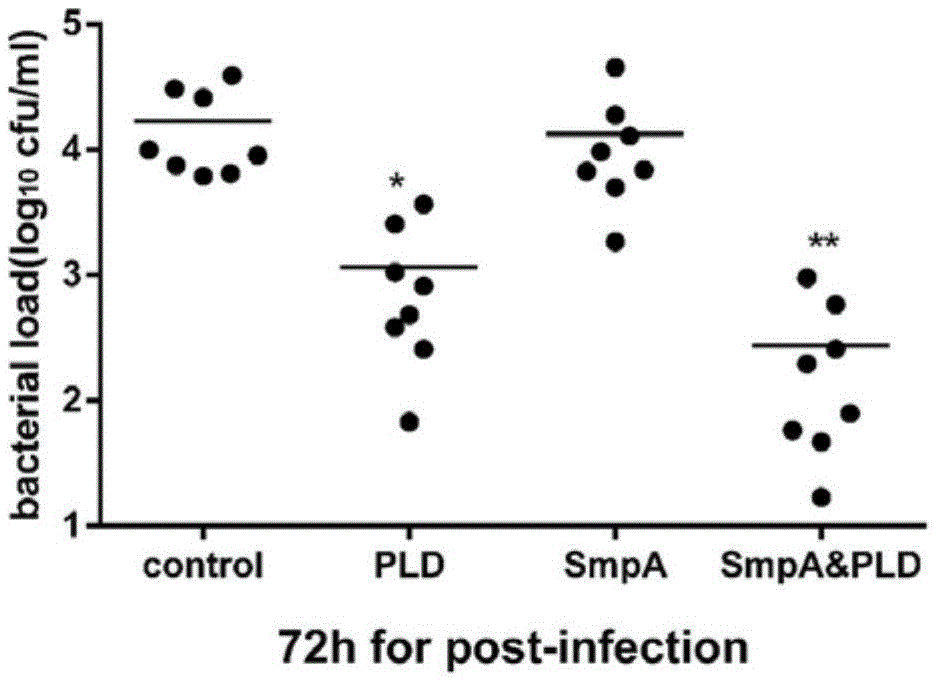
[0069] Recombinant SmpA and PLD were mixed with 1% aluminum hydroxide solution at a mass ratio of 5 / 25 / 125μg each at a mass ratio of 1:1, and the mixed solution was used for 3 subcutaneous injections on day 0, day 7, and day 21. Diabetic mice are kept in an SPF environment. Two weeks after the last immunization, blood was collected for antibody level ELISA. The antigen was coated with 10μg of recombinant SmpA and PLD during ELISA. The test results confirmed that the antibody titer reached 10 3 -10 6 , Confirmed the production of high titer specific antibodies, and selected SmpA25μg and PLD5μg as the vaccine dose ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com