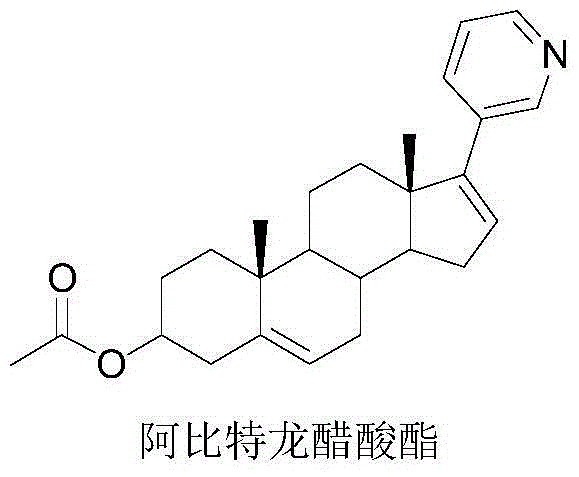
Synthesis method of 17-(3-pyridyl)-androst-4, 16-diene-3beta-ol acetate
A technology of pyridyl and androster, which is applied in the field of synthesizing 17--androster-4, which can solve the problems of high price of diethyl-3-pyridine borane, low purity of abiraterone acetate, complicated operation of pyridine zinc reagent, etc. problems, to achieve the effects of easy large-scale production, short reaction steps, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
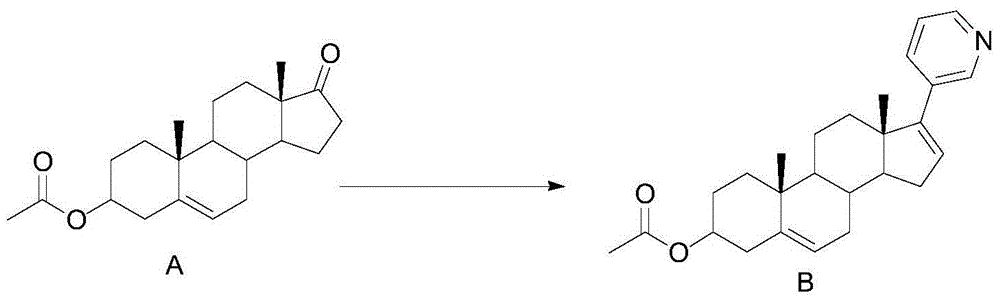
[0036] Embodiment 1: compound B synthesis
[0037]
[0038] Dissolve 30g (0.091mol) of compound A in 300ml of isopropyl ether, cool down to 0°C, add 90.5g (0.100mol) of 3-bromopyridine Grignard reagent dropwise, raise the temperature to room temperature for 18h, and use The reaction was quenched with saturated ammonium chloride aqueous solution, and the isopropyl ether was distilled off under reduced pressure. After evaporation, the mixture was extracted three times with dichloromethane. The organic layers were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate. Remove dichloromethane by distillation under reduced pressure. After the distillation is complete, add 300ml of methanol to the residue, add 23.3g (0.091mol) of Burgess reagent under stirring, heat and reflux for 1h, and add 23.3g (0.091mol) of Burgess reagent , TLC monitors the completion of the reaction, cooling, quenching the reaction with ice water, separating the layers, extractin...
Embodiment 2
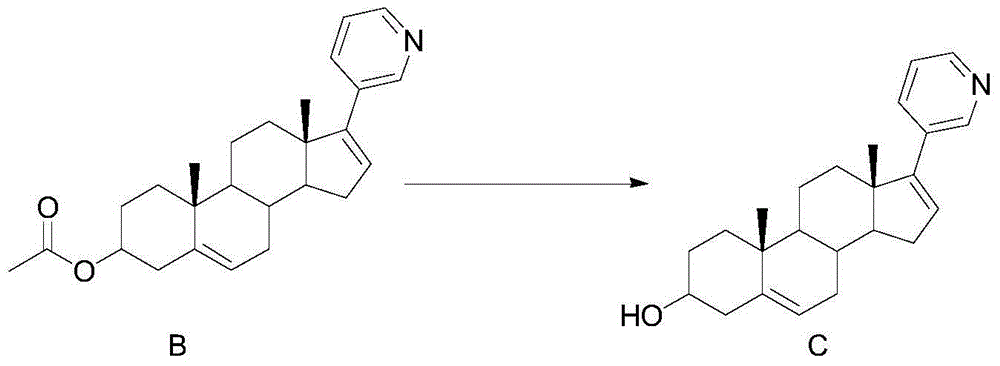
[0039] Embodiment 2: the synthesis of compound C
[0040]
[0041] Dissolve 20g (0.051mol) of compound B in 100ml of ethanol, add 4.08g (0.102mol) of sodium hydroxide in 10ml of aqueous solution to the system, react at room temperature for 2 hours, monitor the completion of the reaction by TLC, filter, and wash the filter cake with water until neutral, 50 °C and dried to obtain 17.1 g of compound C with a yield of 95.8%.
Embodiment 3
[0042] Embodiment 3: Compound C is refined
[0043]
[0044] Add 15g (0.042mol) of compound C to 60ml of isopropanol, heat to reflux, dissolve, cool down to 0°C, crystallize for 1h, filter, wash the filter cake with isopropanol, and dry at 50°C to obtain 12.3g of compound C, Yield 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com