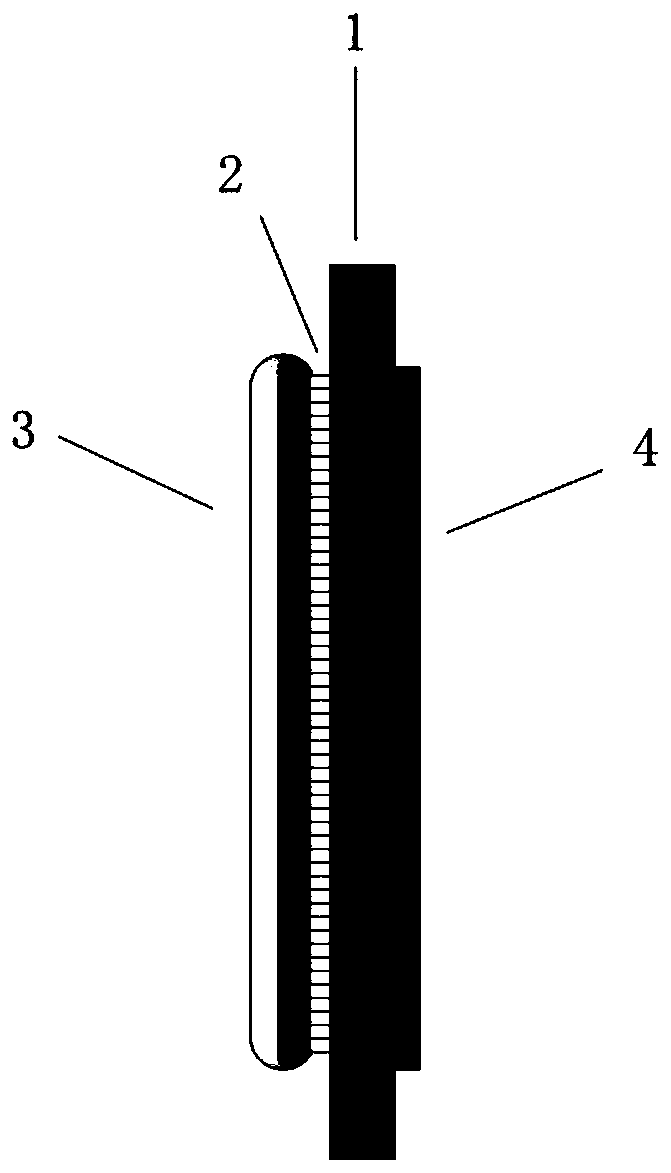
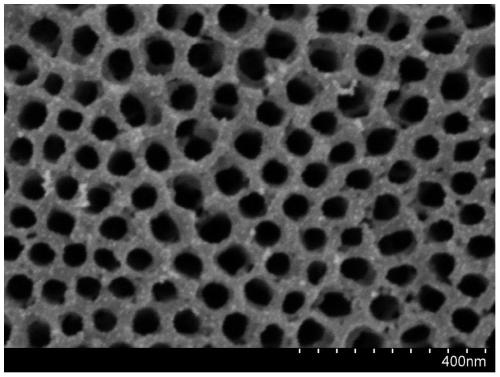
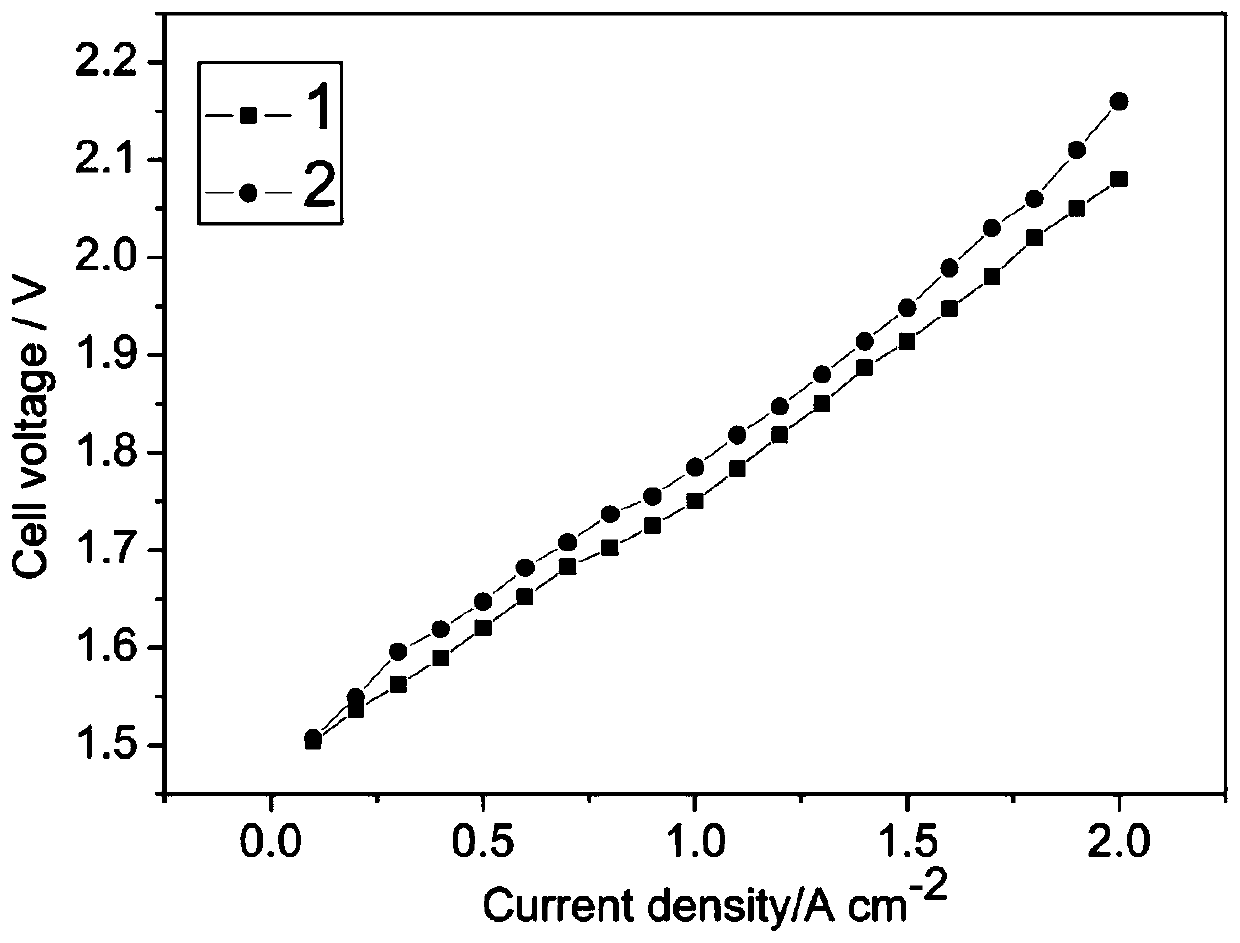
A titanium-based hydrogen evolution electrode for solid polymer water electrolyzer and preparation method thereof
A technology of solid polymers and water electrolyzers, applied in the direction of nanotechnology, electrodes, electrolytic components, etc. for materials and surface science, can solve the problems of reducing the cost of water electrolyzers, large contact resistance, small reaction area, etc., to achieve Simplify the structure and prepare the assembly process, strengthen the electronic conductivity, and increase the effect of the contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Take a porous titanium plate with a porosity of 20%, a powder particle size of 20 microns, and a thickness of 1 mm, cut to a size of 3 cm*1 cm, and place it in 10% hydrochloric acid for heating and etching for 10 minutes to remove the surface oxides and then rinsed several times with deionized water. The obtained porous titanium plate is ultrasonically cleaned in anhydrous ethanol and deionized water for 5 minutes in sequence, and the above is the pretreatment process of the porous titanium plate.
[0033](2) The preparation of TiO nanotube arrays adopts a two-electrode system. The porous titanium plate after the pretreatment was used as the positive electrode, the graphite electrode was used as the negative electrode, 25 ml of 0.5% hydrofluoric acid aqueous solution was added as the electrolyte, and the electrode spacing was 1.5 cm, and then a voltage of 15 V was applied for oxidation for 1 hour, and then taken out. Then, rinsed with pure water several times, and ...
Embodiment 2
[0042] The porous titanium plate used in step (1) has a porosity of 30%, a particle size of 50 microns and a thickness of 1 mm. The cathode current density applied in step (3) is 5mA / cm 2 , the concentration of sodium sulfate solution is 1M, the polarization time is 20 minutes, in step (4), the concentration of platinum precursor is 0.5g / L, the concentration of sodium borohydride solution is 0.01M, the reduction time is 30min, the platinum loading 2mg / cm 2 . The rest of the preparation process of titanium oxide nanotubes, Nafion polymer solution components, loading, water electrolyzer assembly process and performance testing methods are all the same as in Example 1. It has been determined that the water electrolyzer is at 1A / cm 2 , the single-slot voltage at 80 degrees is 1.798V.
Embodiment 3
[0044] The porous titanium plate used in step (1) has a porosity of 20%, a particle size of 30 microns and a thickness of 0.7 mm. The voltage applied in step (2) is 30V, the time is 2 hours, and then sintered at 450° C. for 1 hour. At this time, the diameter of the titanium oxide nanotube is 150 nanometers and the height is 2000 nanometers. In step (4), the concentration of platinum precursor is 5g / L, the concentration of sodium borohydride solution is 0.1M, the reduction time is 10min, and the platinum loading is 0.05mg / cm 2 . Nafion loading is 1mg / cm in step (5) 2 , the rest of the titanium oxide nanotube conductivity enhancement process, the water electrolyzer assembly process and the performance testing method are all the same as in Example 1. It has been determined that the water electrolyzer is at 1A / cm 2 , the single-slot voltage at 80 degrees is 1.822V.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com