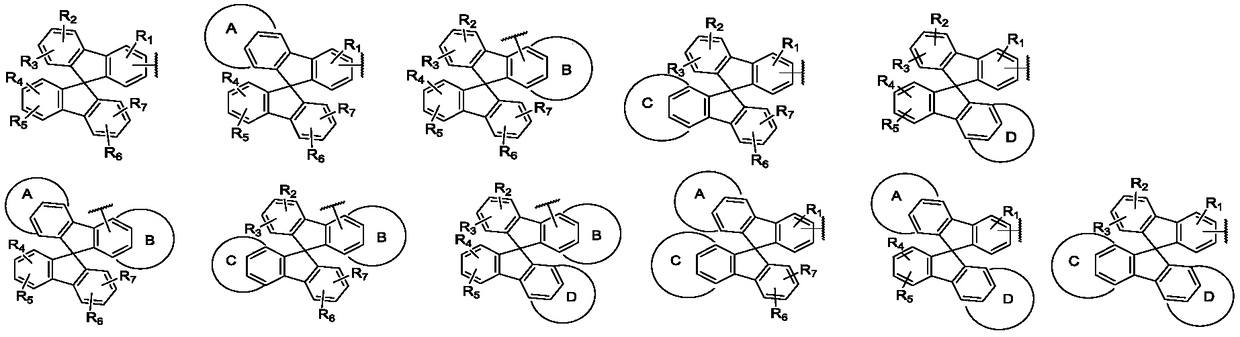
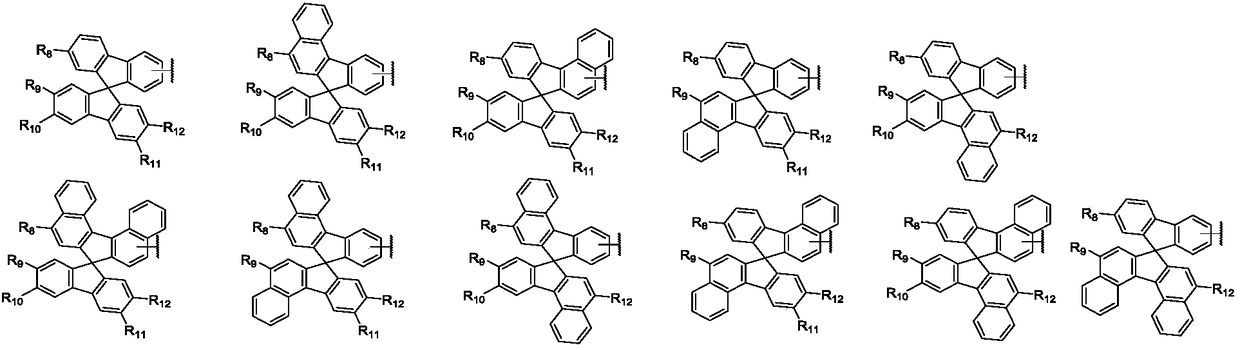
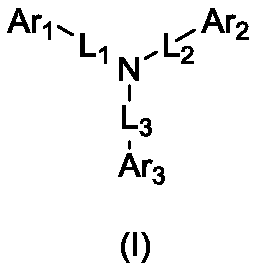Aromatic amine compound containing 9, 9'-spirobifluorene and dibenzofuran and organic electroluminescent device thereof
A technology of benzofuran and spirobifluorene, which is applied in the field of organic photoelectric materials, can solve the problems of shortened lifespan, color purity and low efficiency of organic electrical components, and achieve charge balance, hole transport ability and stability improvement, The effect of lowering the driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the preparation of bromide B
[0061]
[0062]
[0063] Preparation of Bromide B-1:
[0064]
[0065] a) Under nitrogen atmosphere, dissolve methyl 2-iodo-3-bromobenzoate (10.0g, 30mmol), phenylboronic acid (3.66g, 30mmol) and tetrakis(triphenylphosphine)palladium (1.7g, 1.46mmol) In 100ml of THF, stirred for 30 minutes, then added dropwise 50ml of potassium carbonate aqueous solution (1.63g, 11.18mmol) within 20 minutes, refluxed overnight, washed with water, extracted with dichloromethane, evaporated the organic solvent; b) the resulting solid (8.5 g, 255.0mmol) and sodium hydroxide (1.2g, 30mmol) were dissolved in 100ml ethanol, refluxed for 6 hours, cooled to room temperature, neutralized with 2M hydrochloric acid, filtered, and the resulting solid was recrystallized in ethanol; c) obtained Crystals (10g, 30mmol) were dissolved in 300ml methanesulfonic acid, stirred at 30°C for 24 hours, poured into ice water, filtered, washed with water, dri...
Embodiment 2
[0071] Embodiment 2: the preparation of bromide C
[0072]
[0073] Preparation of Bromide C-1:
[0074]
[0075] a) 1-bromo-2-methoxynaphthalene (4.74g, 20mmol), pinacol diboronate (6.22g, 24mmol), 1,1'-bis(diphenylphosphine)-ferrocene- Palladium dichloride (II) dichloromethane complex (0.49g, 0.6mmol), potassium acetate (5.90g, 60mmol) and 79ml of toluene were reacted under reflux for 16 hours, cooled, added 26ml of water, stirred for 30 minutes, separated The organic phase was filtered through a short celite bed, and then the organic solvent was evaporated, and the resulting crude product was recrystallized in heptane / toluene; the solid (4.26 g, 15 mmol) obtained in the previous step, 1-bromo-2-fluoro- 3-iodobenzene (4.05g, 14.3mmol), tetrakis(triphenylphosphine)palladium (0.35g, 0.3mmol), toluene (43ml), 2M aqueous sodium carbonate solution (21ml) were added to the flask, and the reaction was refluxed for 8 hours. Cool to room temperature, extract with toluene, was...
Embodiment 3
[0081] Embodiment 3: the preparation of aromatic amine compound A
[0082]
[0083] Preparation of Compound A-1:
[0084]
[0085] Add bromobenzene (4.71g, 30mmol), cuprous iodide (0.06g, 0.3mmol), and 45ml of liquid ammonia into the flask, react at 100°C for 18 hours, filter, the liquid is extracted, washed, dried, and the organic solvent is removed , and finally obtain compound A-1.
[0086] Compounds A-2 to A-31 can all be prepared by the above methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com