Optical device with negative thermal expansion substrate and uses therefor
a technology of thermal expansion substrate and optical device, which is applied in the direction of optical elements, cladded optical fibres, instruments, etc., can solve the problems of cte curves that exhibit considerable hysteria, material negative mean lattice expansions that do not require microcracking for negative bulk ceramic ctes are even more limited, and high porosity bodies are generally not useful for industrial applications. , to achieve the effect of reducing particle size, preventing microcracking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 19 to 28
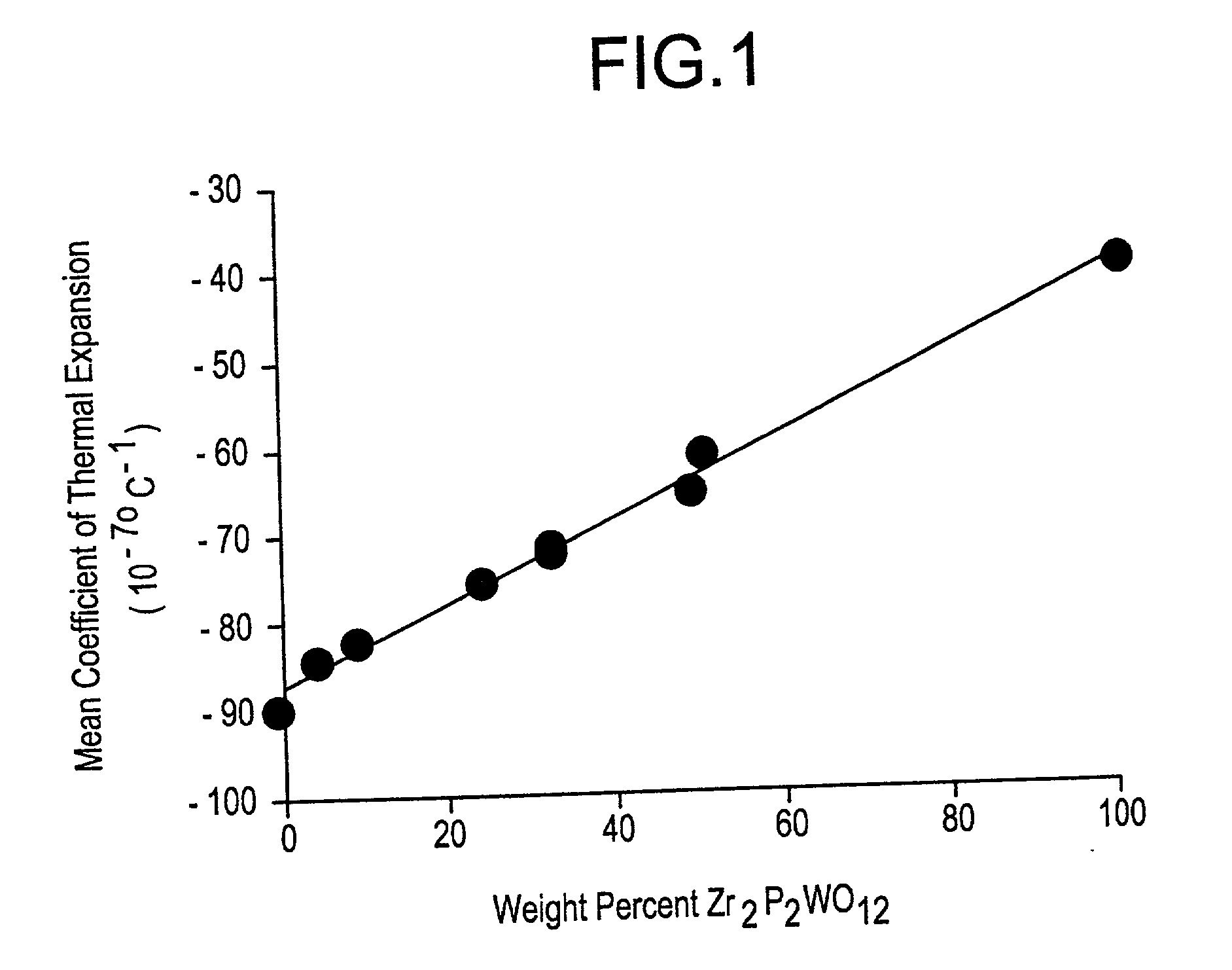
[0057] Powder preparation for Examples 19 and 21-28 was conducted in the same manner as Examples 1-18, with the proportions of the starting materials adjusted to yield Zr.sub.2P.sub.2WO.sub.12 and ZrW.sub.2O.sub.8 in the ratios stated in Table 2. In these examples, the weight percentages of Zr.sub.2P.sub.2WO.sub.12 and ZrW.sub.2O.sub.8 are relative to the sum of the weights of the Zr.sub.2P.sub.2WO.sub.12 and ZrW.sub.2O.sub.8 components only. Weight percent of additive is relative to total weight percent of starting material.
2TABLE 2 Experimental data for Zr.sub.2P.sub.2WO.sub.12 ceramics with various amounts of ZrW.sub.2O.sub.8. Heating rate Weight Weightabove Example % % Weight % Sample 900.degree. C. Soak Soak Number Zr.sub.2P.sub.2WO.sub.12 ZrW.sub.2O.sub.8 Additive Additive Geometry (.degree. C. / hr) Temp Time 19 100 0 0.00 none 7.6 cm 200 1150 4 bar 20 50 50 0.00 none 7.6 cm 100 1150 4 bar 21 49 51 0.00 none 7.6 cm 200 1150 4 bar 22 32 68 0.00 none 7.6 cm 100 1150 4 bar 23 32 6...
example 20
[0058] prepared by precipitation from aqueous solutions of ZrOCl.sub.2.8H.sub.2O, NH.sub.4H.sub.2PO.sub.4, and H.sub.2WO.sub.4. According to this method, 79.18 grams of zirconyl chloride (previously assayed to be 2.739.times.10.sup.-3 moles Zr per gram of salt) was dissolved in 161 grams of water to form the first solution. A second solution was formed by dissolving 16.63 grams of ammonium dihydrogen phosphate in 73 grams of water. A third solution was made by dissolving 54.19 grams of tungstic acid in 152 grams of water and 54 grams of 14.8 N ammonium hydroxide solution, and heating to 95.degree. C. The tungsten and phosphate solutions were mixed together and then added to the zirconium solution. More ammonium hydroxide was added to the mixture to deflocculate the suspension. The slurry was stirred and heated to 85.degree. C. until dry. The solid was then calcined at 900.degree. C. for 4 hours and subsequently crushed and vibratory milled in isopropanol for 14 hours with zirconia m...
examples 29 to 88
[0062] Powder preparation for Examples 29 to 88 was conducted in the same manner as Examples 1-18, with the proportions of the starting materials adjusted to yield Zr.sub.2P.sub.2WO.sub.12 and ZrW.sub.2O.sub.8 in the ratios stated in Table 2, with the following exceptions: Example 68 was prepared using ZrO.sub.2, WO.sub.3, and a ZrP.sub.2O.sub.7 powder prepared by calcination of acid zirconium phosphate at 1050.degree. C. for 4 hours, and Examples 69 and 73-78 were prepared from ZrO.sub.2, WO.sub.3, and pre-reacted Zr.sub.2P.sub.2WO.sub.12. The pre-reacted Zr.sub.2P.sub.2WO.sub.12 was formed by dry ball milling a mixture of ZrO.sub.2, WO.sub.3 and acid zirconium phosphate in the appropriate ratios and calcining the mixture at 1050.degree. C. for 4 hours. Also, the powders for Examples 72 to 78 were milled in water instead of isopropanol. Examples 29 to 88 were formulated to yield ceramics with coefficients of thermal expansion between -65 and -85.times.10.sup.-7.deg-ree. C..sup.-1.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com