Methods and materials for the synthesis of organic products
a technology of organic products and synthesis methods, applied in the field of methods and materials involved in the can solve the problems of difficult and expensive, and achieve the effects of convenient and efficient production of organic products, rapid growth and efficient production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
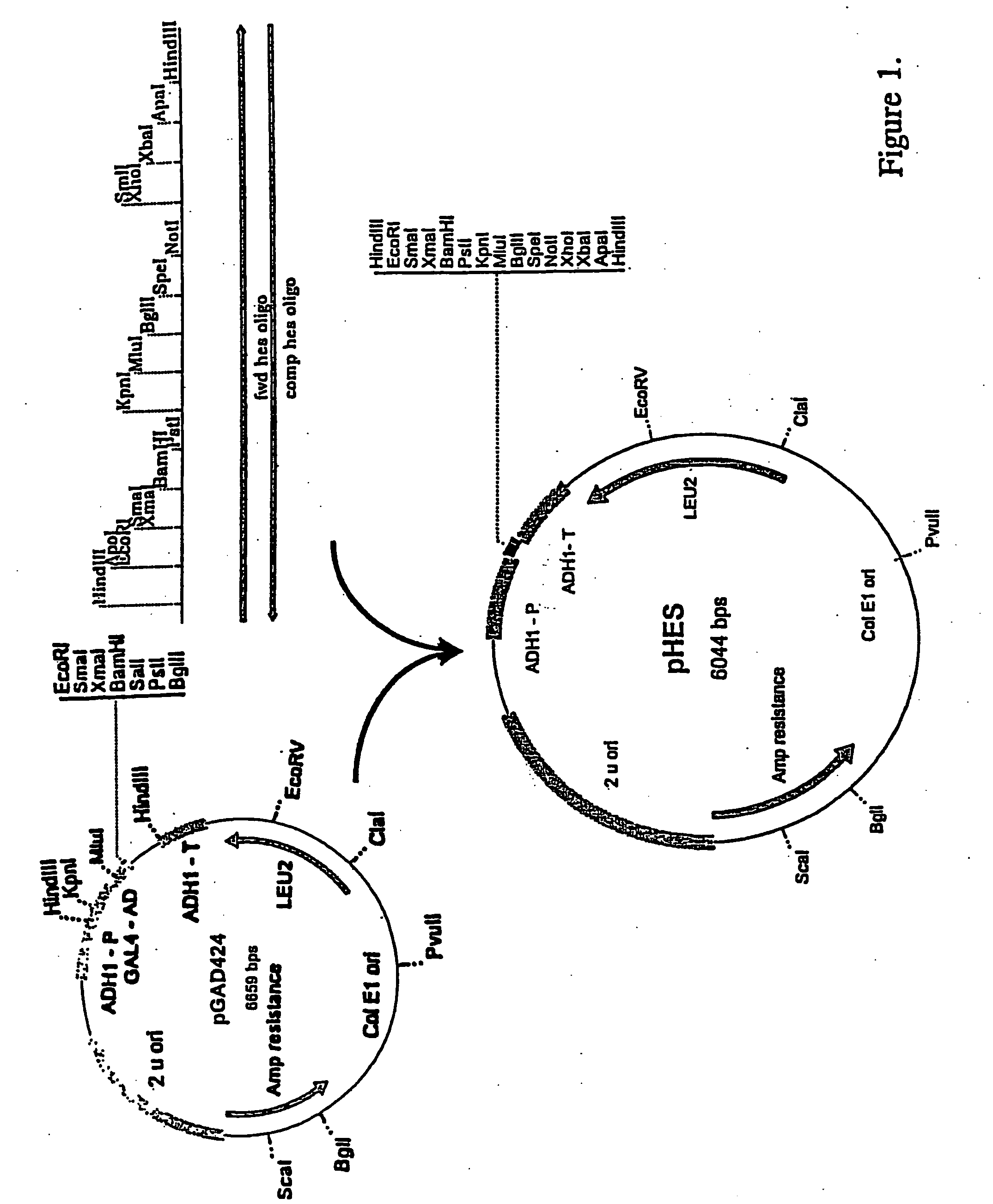
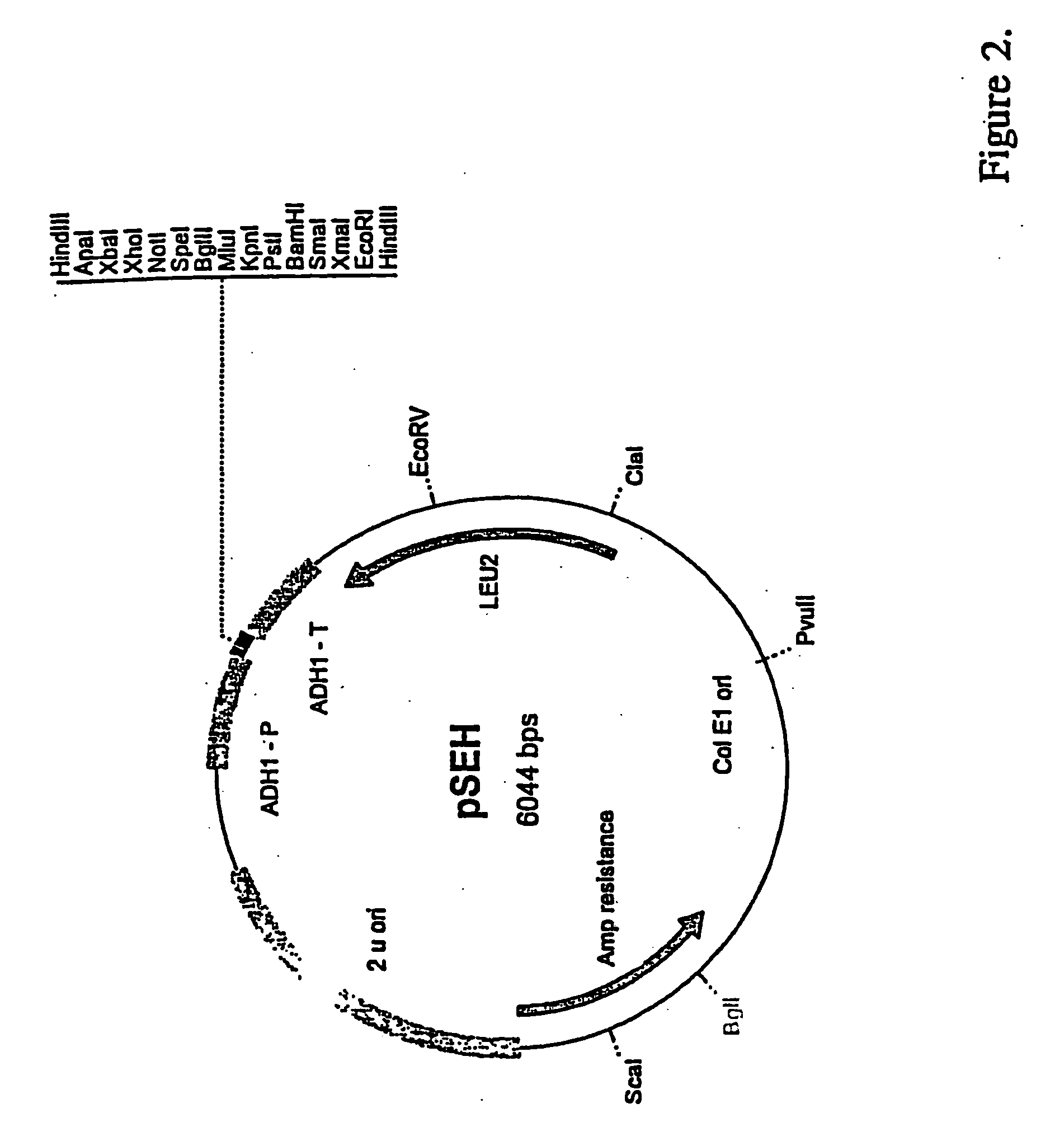
Recombinant Plasmid pHES / pSEH
[0138] 0.5 ug of plasmid pGAD424 described by Chien et al. (Proc. Natl. Acad. Sci., 88:9578-9582 (1991)) was digested with the restriction enzyme HindIII. The digested mixture was separated by gel electrophoresis on a 0.8% agarose gel using TBE buffer. A 5.9 kbp fragment was then purified from the gel as described in Sambrook et al., (ibid.). A complementary pair of 92 bp synthetic oligomers with multiple restriction enzyme recognition sites was designed. The first was designated fwd HES oligo and has the following sequence:
5′-CCCAAGCTTGAATTCCCCGGGGGATCCCTGCAGGGTACCACGCGTAGA TCTACTAGTGCGGCCGCCTCGAGTCTAGAGGGCCCAAGCTTGGG-3′ (SEQ ID NO: 1). The second was designated camp hes oligo and has the following sequence:
[0139] 5′-CCAAGCTTGGGCCCTCTAGACTCGAGGCGGCCGCACTAGTAGATCTAC GCGTGGTACCCTGCAGGGATCCCCCGGGGAATTCAAGCTTGGG-3′ (SEQ ID NO:2). 500 nmoles of the two complementary oligomers were annealed to each other by boiling for ten minutes and cooling gradually to...
example 2
PCR Amplification of Nucleic Acid Encoding Lactate Dehydrogenase from Lactobacillus helveticus and Pediococcus acidilactici
[0140] Genomic DNA was isolated from overnight cultures of Lactobacillus helveticus (ATCC 10797) and Pediococcus acidilactici (ATCC 25741) using PUREGENE® genomic DNA isolation kit (Gentra systems, Minneapolis, Minn.). PCR primers were designed to isolate lactate dehydrogenase-encoding nucleic acid from L. helveticus (lh-ldh oligos) and P. acidilactici (pa-ldh oligos) genomic DNA. These primers were designed based on the available gene sequences for lactate dehydrogenases in the Genbank databases, and have the following sequences:
5′ lh-ldh, 5′-CCGGGATCCATGGCAAGAGAGGAAAAACCTC-3′ (SEQ ID NO:3);
3′ lh-ldh, 5-CCAAGATCTTTATTGACGAACCTTAACGCCAG-3′ (SEQ ID NO:4);
5′ pa-ldh: 5′-CCGGGATCCATGTCTAATATTCAAAATCATCAAAAAG-3′ (SEQ ID NO:5); and
[0141] 3′ pa-ldh, 5′-CCAAGATCTTTATTTGTCTTGTTTTTCAGCAAG-3′ (SEQ ID NO:6). The primers were optimized using Primer Designer software ...
example 3
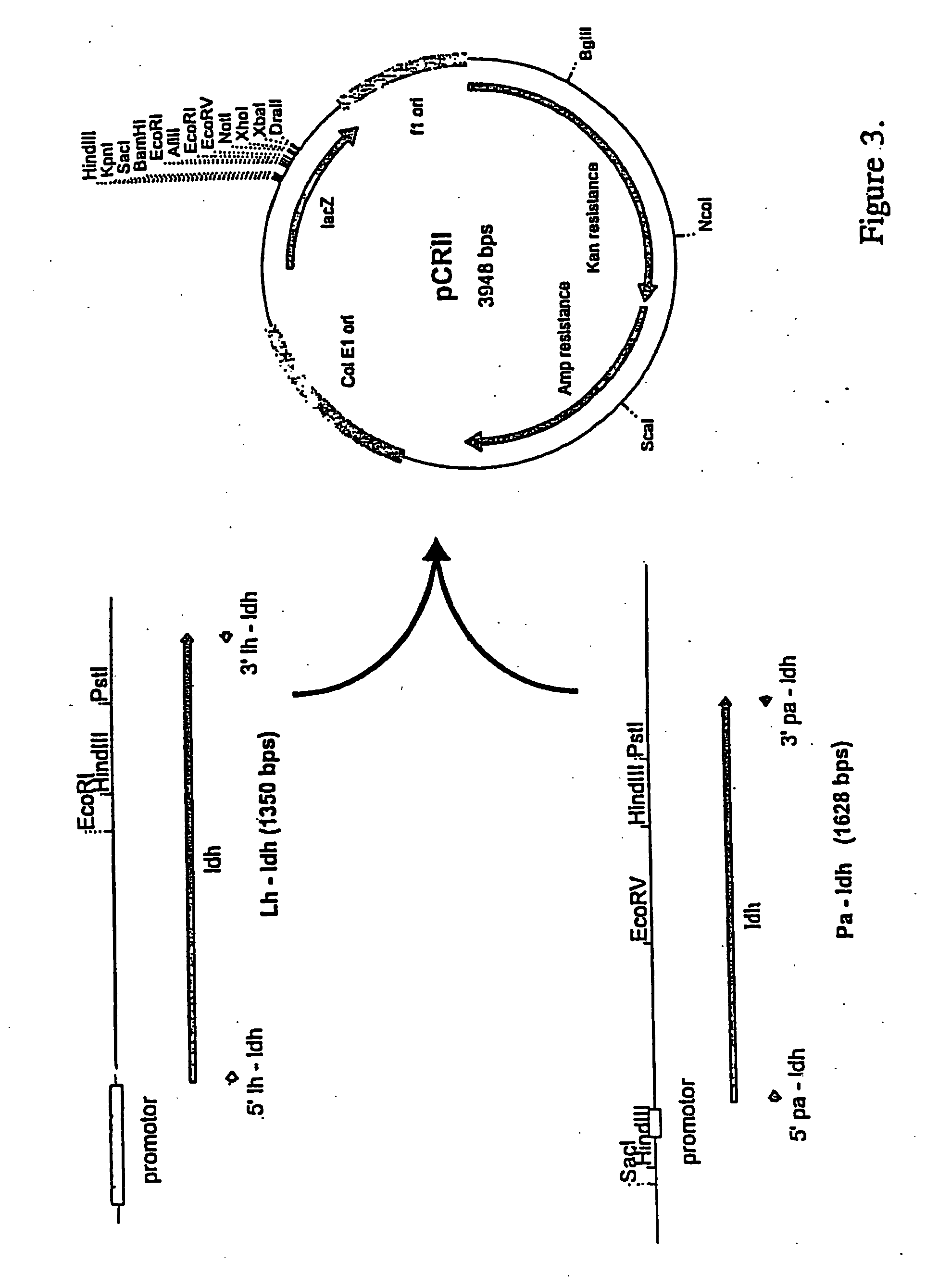
Cloning of L. helveticus and P. acidilactici LDH Genes into pCRII Vector
[0148] PCR amplified LDH DNA products were ligated with pCRII vectors (FIGS. 3 and 4) using the TA cloning kit obtained from Invitrogen (Carlsbad, Calif.). The ligation mixture was then used to transform E. coli DH10B using methods described in Sambrook et al. (ibid.). The pCRII vectors supplied with the kit allowed for quick cloning of the PCR products according the manufacture's instructions. The pCRII vectors with the LDH genes from L. helveticus and P. acidilactici are depicted in FIG. 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com