Method for promoting expression of insulin receptor substrate-2
a technology of insulin receptor and substrate, which is applied in the direction of drug composition, peptide/protein ingredient, metabolic disorder, etc., can solve the problems of serious diabetic complications, serious human health harm, and impact on the quality of life of patients, so as to reduce the apoptosis of pancreatic islet cells, reduce inflammation, and reduce the damage to the pancreas.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
en Promotes Expression of Insulin Receptor Substrate 2 (IRS-2) in Pancreatic Islet of 18-Week-Old Diabetic Mice
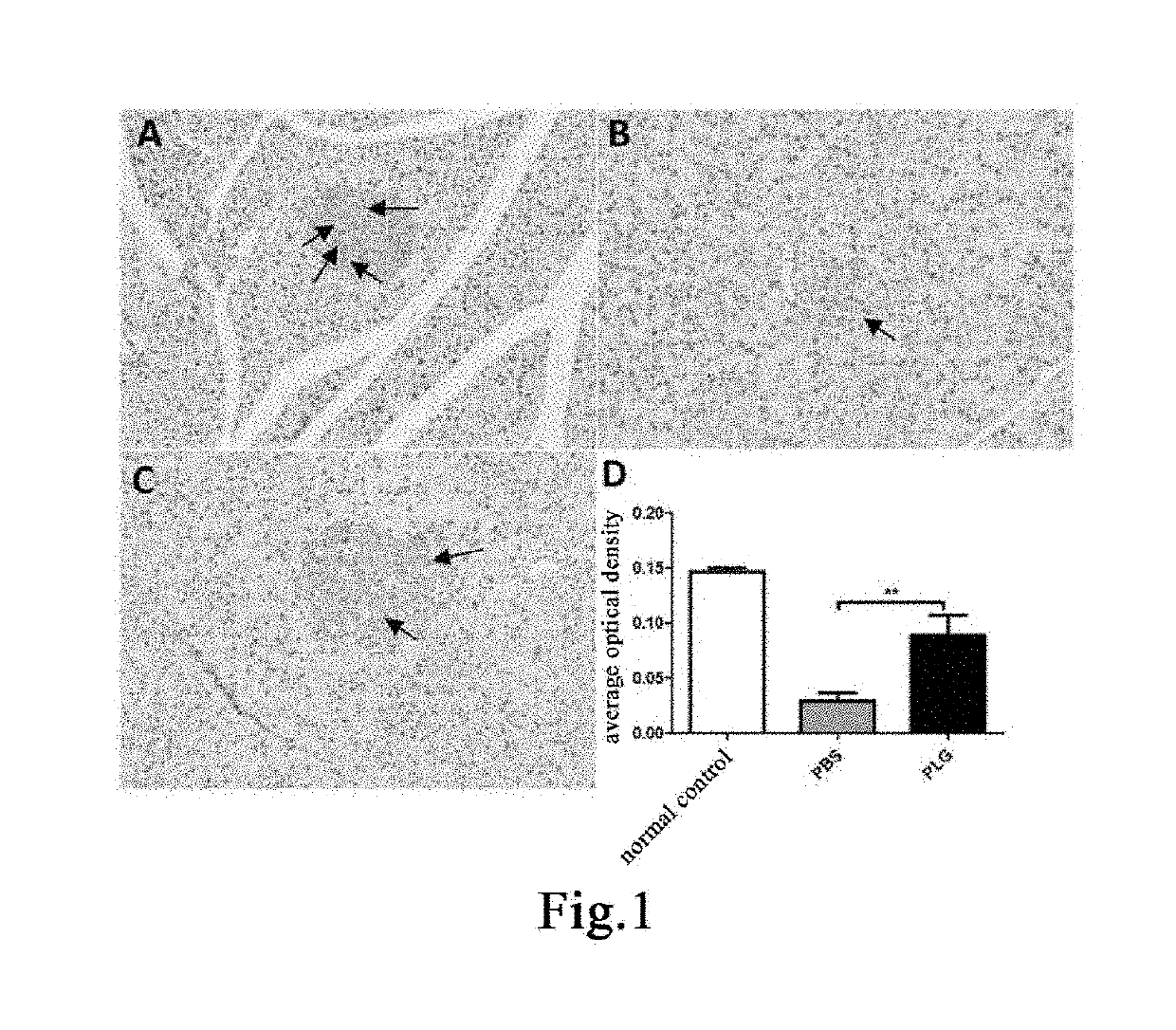
[0203]Seven male db / db mice and three male db / m mice, 18 weeks old, were weighed and the db / db mice were randomly divided, according to body weight, into two groups, a group of 3 mice administered with plasminogen and a control group of 4 mice administered with vehicle PBS, on the day the experiment started that was recorded as day 0; in addition, the db / m mice were used as a normal control group. Starting from day 1, plasminogen or PBS was administered. The mice in the group administered with plasminogen were injected with human plasminogen at a dose of 2 mg / 0.2 mL / mouse / day via the tail vein, and the mice in the control group administered with vehicle PBS were injected with an equal volume of PBS via the tail vein, both lasting for 35 consecutive days. On day 36, the mice were sacrificed, and the pancreas was taken and fixed in 4% paraformaldehyde. The fixed pancreas tiss...
example 2
en Lowers Blood Glucose in Diabetic Mice
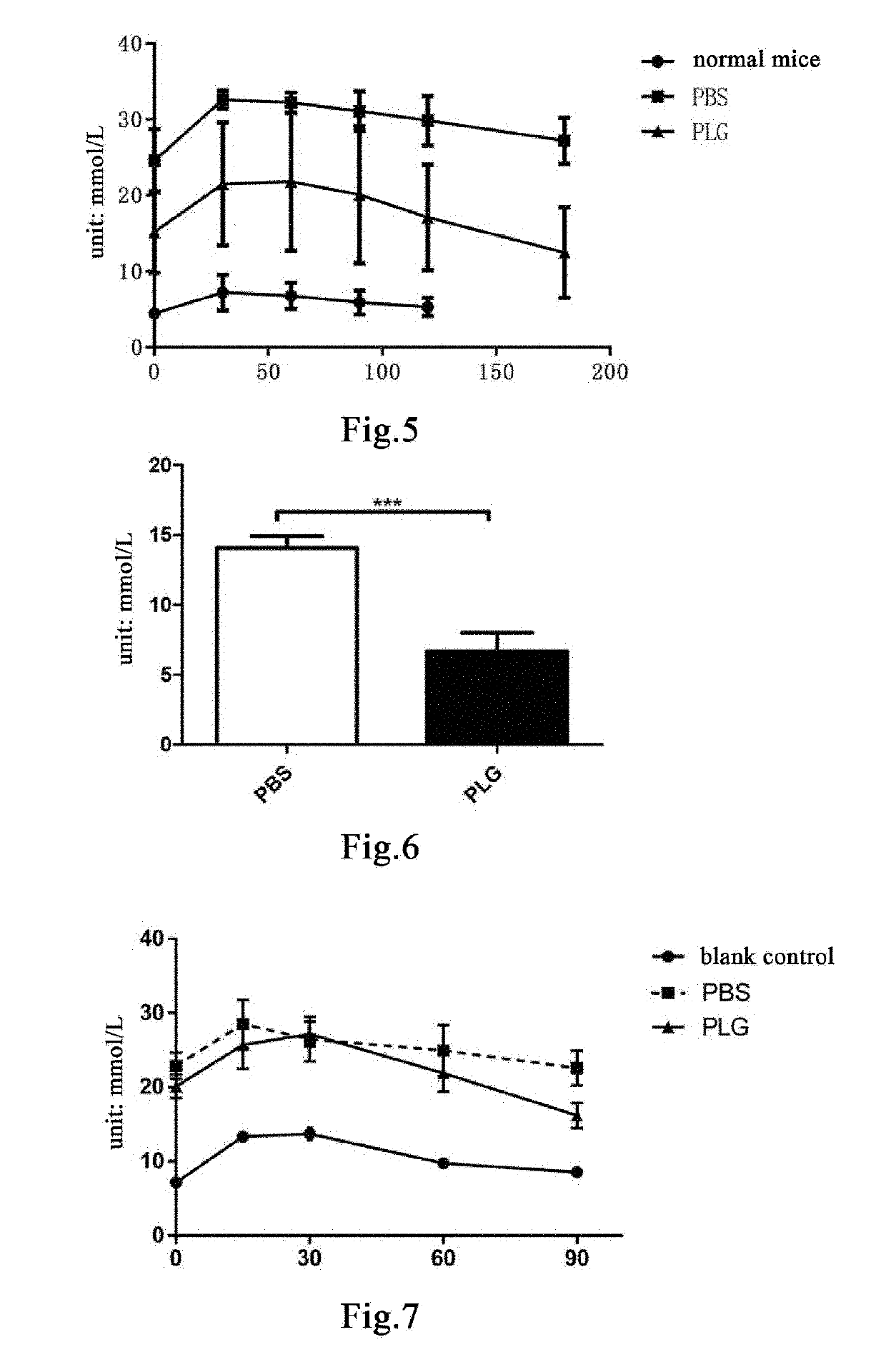
[0206]Eight 24- to 25-week-old male db / db mice were randomly divided into two groups, a group of 5 mice administered with plasminogen, and a control group of 3 mice administered with vehicle PBS. The mice were weighed and grouped on the day when the experiment began, i.e. day 0. Starting from the 1st day, plasminogen or PBS was administered. The group administered with plasminogen was injected with human plasminogen at a dose of 2 mg / 0.2 mL / mouse / day via the tail vein, and the control group administered with vehicle PBS was injected with an equal volume of PBS via the tail vein, both lasting for 31 consecutive days. After fasting for 16 hours on days 10 and 31, blood glucose testing was carried out using a blood glucose test paper (Roche, Mannheim, Germany).
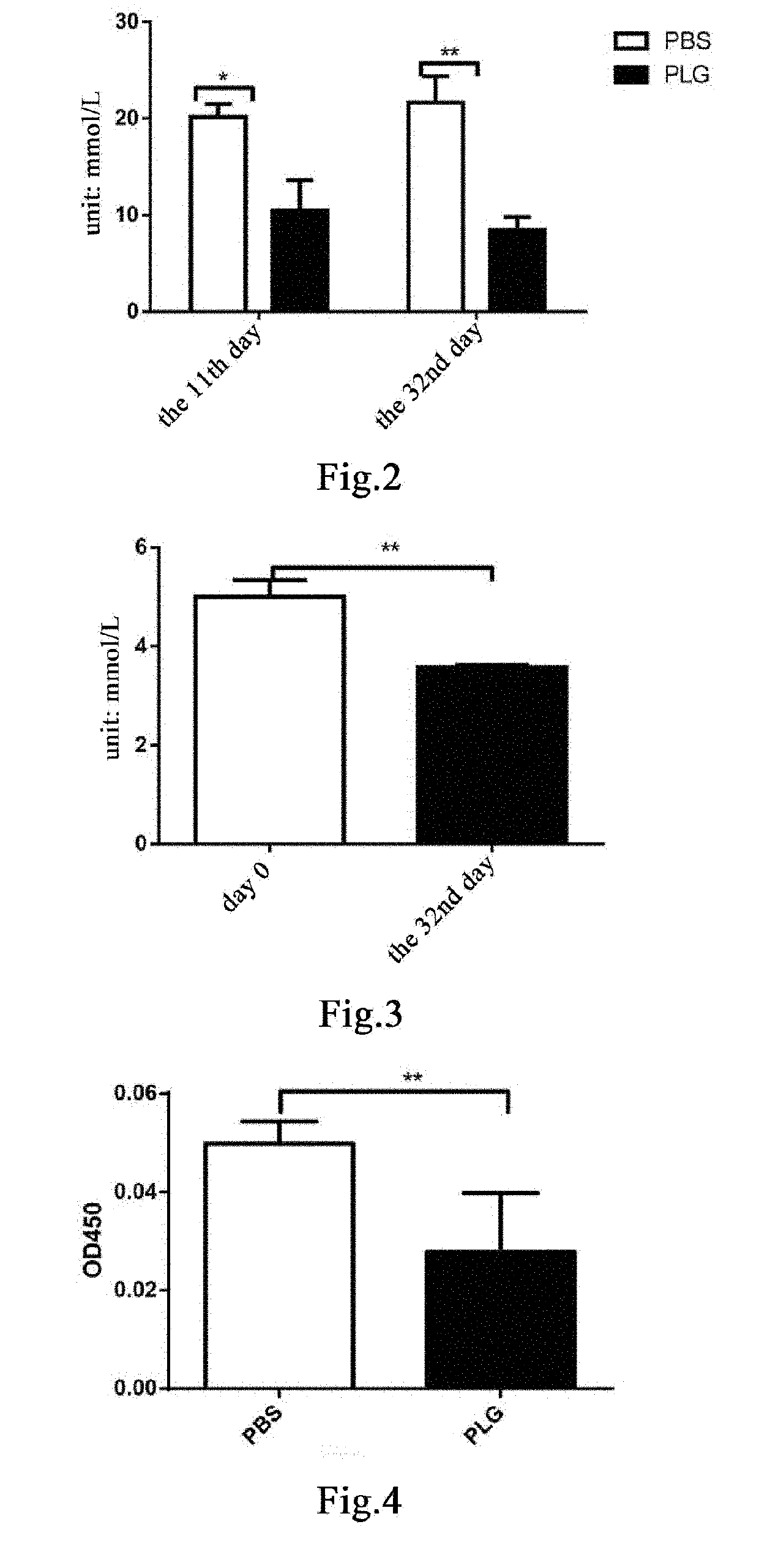
[0207]The results show that the blood glucose level in mice in the group administered with plasminogen was remarkably lower than that in the control group administered with vehicle PBS, and...
example 3
en Lowers Fructosamine Level in Diabetic Mice
[0208]For five 24- to 25-week-old male db / db mice, 50 μl of blood was collected from venous plexus in the eyeballs of each mouse one day before administration, recorded as day 0, for detecting a concentration of serum fructosamine; and starting from day 1, plasminogen is administered for 31 consecutive days. On day 32, blood was taken from the removed eyeballs to detect the concentration of serum fructosamine. The concentration of fructosamine was measured using a fructosamine detection kit (A037-2, Nanjing Jiancheng).
[0209]The concentration of fructosamine reflects the average level of blood glucose within 1 to 3 weeks. The results show that the concentration of serum fructosamine is remarkably decreased after administration of plasminogen, and as compared with that before administration, the statistical difference is extremely significant (FIG. 3). This indicates that plasminogen can effectively reduce blood glucose in diabetic animals....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com