Composition containing bifidobacteria for adjusting intestinal flora and enhancing immunity
A technology that regulates intestinal flora and enhances immunity. It is applied in the direction of drug combination, microbial-based methods, and active ingredients of hydroxyl compounds. Achieve the effect of inhibiting the growth of spoilage bacteria, reducing the culture volume, and inhibiting the change of physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of Bifidobacterium powder
[0042] 1. High-density cultivation
[0043] (1) Bacteria activation: Under aseptic conditions, pick the bacteria in the ampoule tube and inoculate them on liquid culture medium and agar plates, culture them anaerobicly at 37°C for 48 hours, and activate continuously for 3 generations to obtain activated bacteria. .
[0044] Among them, the preparation method of the bifidobacteria medium used in the present invention is as follows: tryptone 5.0g, yeast extract 10.0g, soy peptone 5.0g, glucose 10.0g, L-cysteine hydrochloride 0.5g, vomiting Warm 801.0mL, salt solution 40mL, add water to make the volume to 1000mL, adjust pH 6.8~7.0, sterilize at 121℃ for 20min. The composition of the salt solution is: calcium chloride 0.2g, potassium dihydrogen phosphate 1.0g, magnesium sulfate 0.48g, sodium bicarbonate 10.0g, dipotassium hydrogen phosphate 1.0g, sodium chloride 2.0g, and the volume is made up to 1000 mL with water.
[0045] (2...
Embodiment 2
[0060] Example 2: Composition formula
[0061] Component
[0062] The components of the composition are mixed according to the above-mentioned dosage to make a capsule, 0.25g / capsule, 4 capsules each time, 2 times a day; to make an granule, 1g / packet, 1 bag each time, 2 times a day.
Embodiment 3
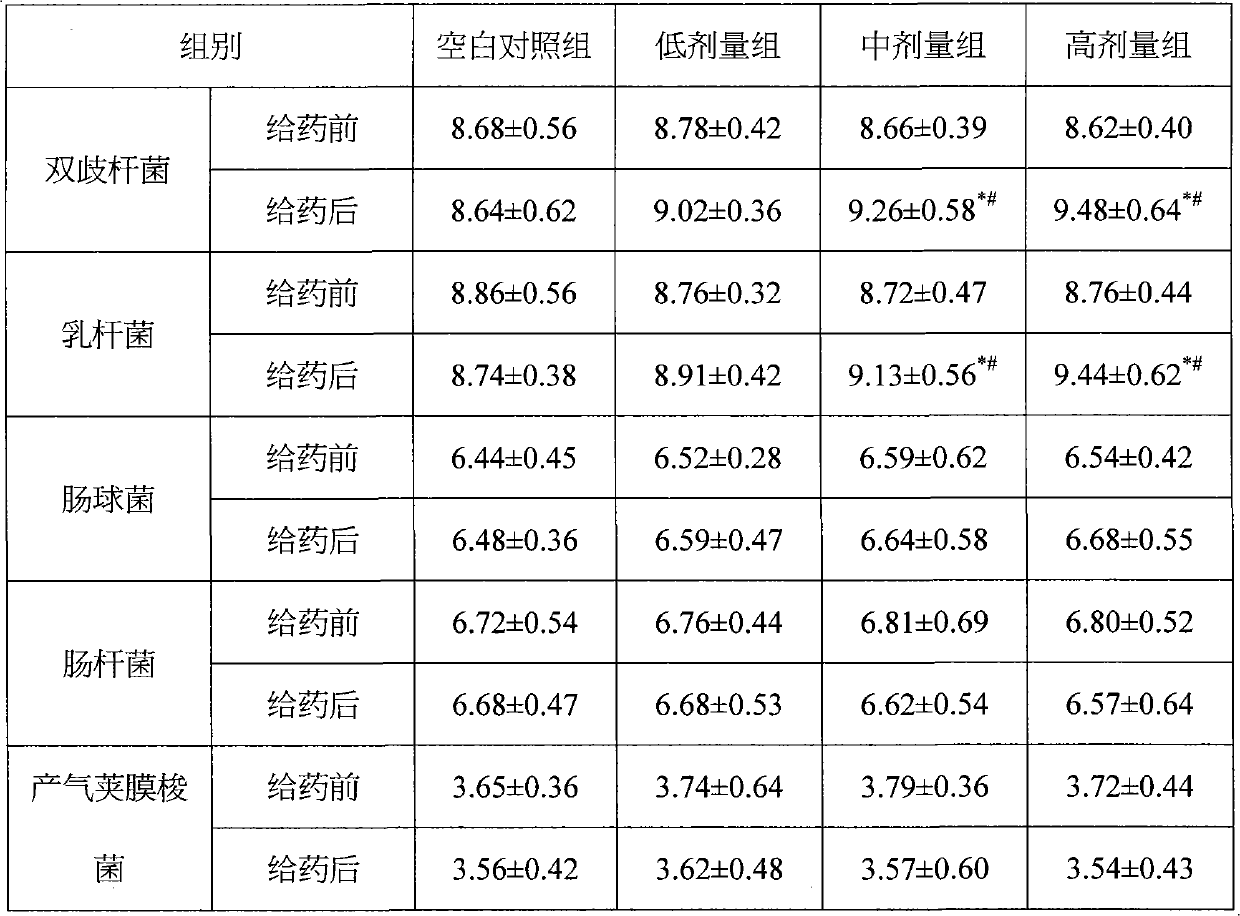
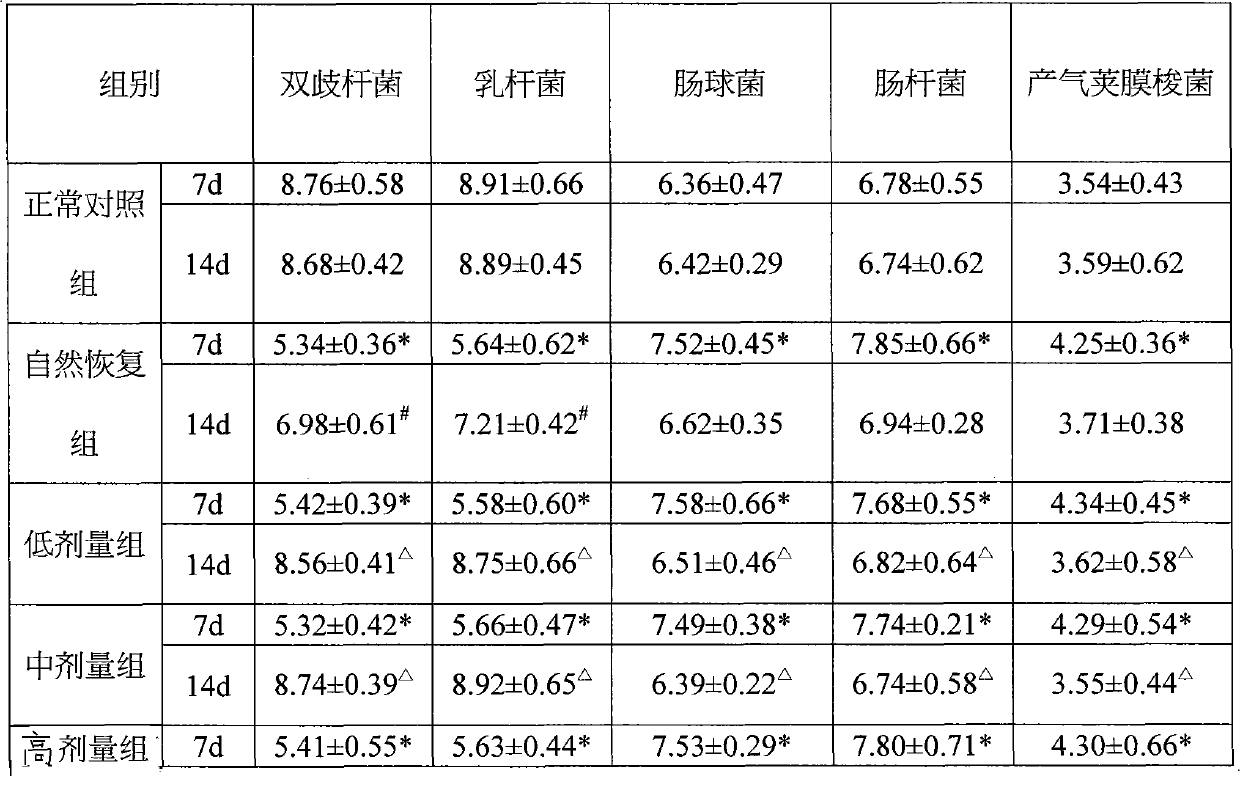
[0063] Example 3: Function test of regulating intestinal flora in normal mice
[0064] Animal grouping and administration: 40 BALB / C inbred mice, male, 6-8 weeks old, 18-22g / mouse, randomly divided into blank control group and composition low, medium and high dose groups, each with 10 only. The low, medium and high dose groups were given the composition 0.17, 0.33, and 1.0 g / kg (equivalent to 5, 10, and 30 times the recommended human dose) by intragastric administration. The blank group was given normal saline once a day for 14 days, and the indicators of body weight, Bifidobacterium, Lactobacillus, Enterococcus, Enterobacter, and Clostridium perfringens were checked regularly.
[0065] Detection method: Before administering the composition, aseptically take 0.1g of mouse feces and dilute it to 10 times serially. -8 , Select the appropriate dilution to inoculate each medium separately. After culturing, the colonies are identified and counted by colony morphology, Gram stain micro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com