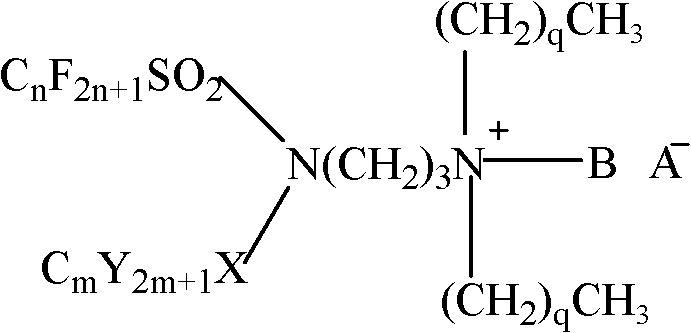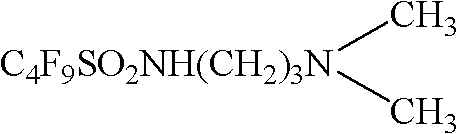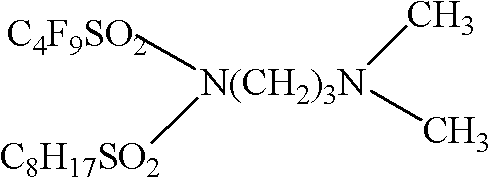Fluorine-containing imine cationic surfactant preparation method and application thereof
A fluorine-containing imine type, surfactant technology, applied in the preparation of sulfonic acid amides, the preparation of organic compounds, the preparation of amino compounds, etc., can solve the problem of refractory organic pollutants, high bioaccumulation and human body multi-organ toxicity and other problems, to achieve the effect of high surface activity, high practical value and excellent surface performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of fluorine-containing imine cationic surfactant.
[0024] Get 1mol perfluorobutylsulfonyl fluoride and 1mol N,N'-dimethyl-1,3-propanediamine for amidation reaction to obtain intermediate (N-[3-(dimethylamino)-propyl ] perfluorobutylsulfonamide), and then carry out amidation reaction with octylsulfonyl chloride to obtain the intermediate (N'-3-(dimethyl)-propyl-(N-perfluorobutylsulfonyl-N- octylsulfonyl)-imine), after recrystallization with dichloromethane, acetonitrile as solvent, reacted with iodomethane to give N′-3-(trimethyl)-propyl-(N-perfluorobutylsulfonyl Acyl-N-octylsulfonyl)-tertiary amine iodide salt; react with hydrogen peroxide to give N'-3-(dimethyl)-propyl-(N-perfluorobutylsulfonyl-N-octylsulfonyl )-amine oxide.
Embodiment 2
[0026] The synthesis of N-[3-(dimethylamino)-propyl] perfluorobutylsulfonamide in embodiment 1
[0027] The synthesis process is as follows: In a three-necked round bottom flask equipped with a magnet, add 100ml of benzene, 1mol of triethylamine and 1mol of N,N'-dimethyl-1,3-propanediamine, and drop 1mol of Perfluorobutylsulfonyl fluoride, then reacted for 8 hours, suction filtered, and recrystallized with dichloromethane to obtain white crystals, the chemical expression of which is:
[0028]
[0029] MS (623.9); F 19 NMR: -85.952, -115.049, -123.705, -128.212; H 1 NMR: 2.322 (6H, -CH 3 ), 3.502 (2H, -CH 2 -), 2.617 (2H, -CH 2 -,), 1.766 (2H, -CH 2 -), 3.488 (-NH).
Embodiment 3
[0031] Synthesis of N'-3-(dimethyl)-propyl-(N-perfluorobutylsulfonyl-N-octylsulfonyl)-imine in Example 1
[0032] The synthesis process is as follows: In a three-neck round bottom flask equipped with a magnet, add 100ml of benzene, 1mol of triethylamine and 1mol of N-[3-(dimethylammonia)-propyl]perfluorobutylsulfonamide, and place in an ice-water bath Next, 1mol octylsulfonyl chloride was added dropwise, then reacted for 6 hours, filtered with suction, and recrystallized with dichloromethane to obtain white crystals, the expression of the product was:
[0033]
[0034] MS(561.2); F 19 NMR: -85.932, -115.041, -123.694, -128.022; H 1 NMR: 3.419 (2H, -CH 2 -), 2.316 (6H, -CH 3 ), 2.608 (2H, -CH 2 -,), 1.721 (2H, -CH 2 -), 3.412 (2H, -CH 2 -), 1.856 (2H, -CH 2 -), 1.294 (10H, -CH 2 -,), 1.022 (3H, -CH 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com