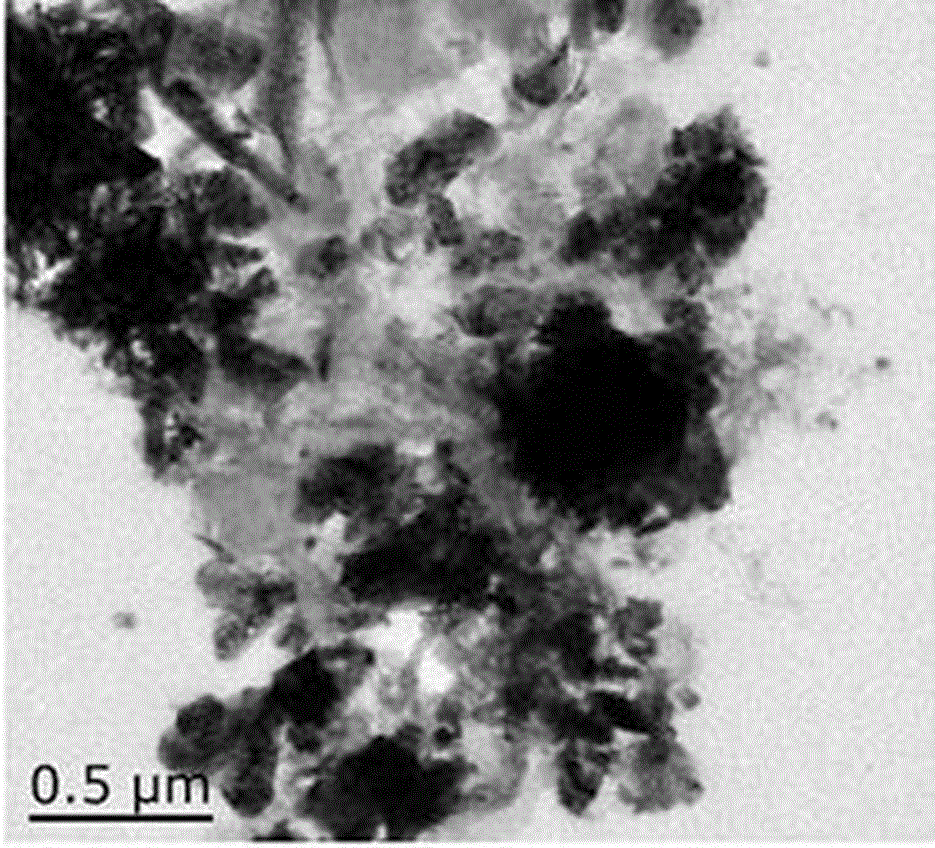
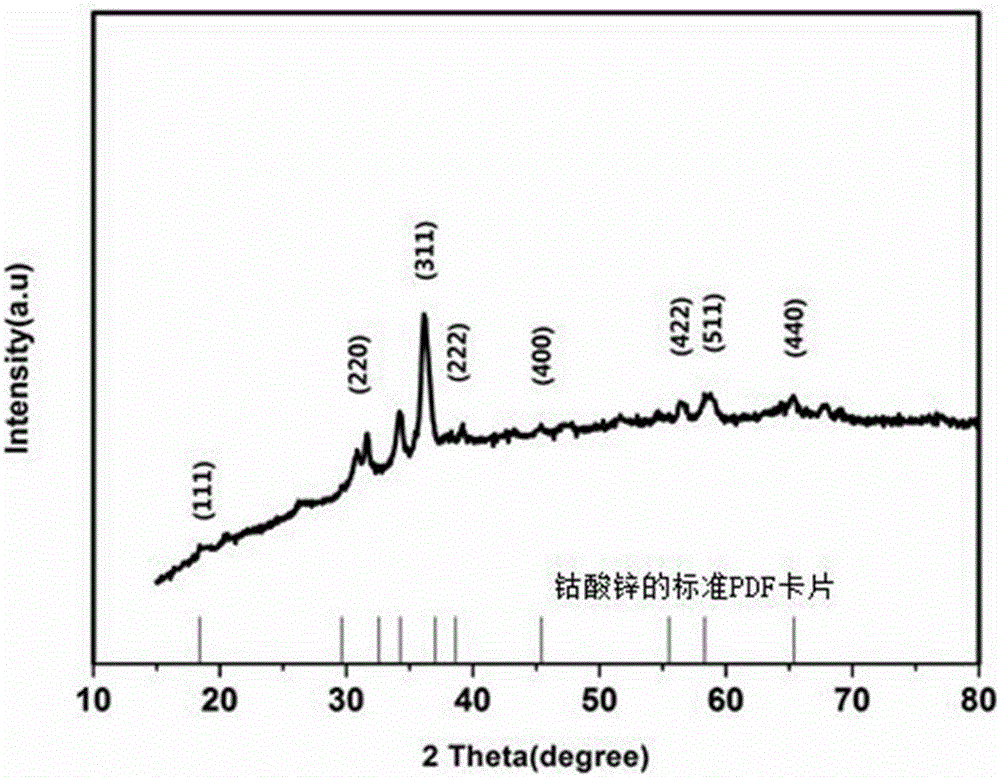
Preparation method and application of nitrogen-doped graphene/nitrogen-doped carbon nanotube/zinc cobaltite composite material
A technology of nitrogen-doped graphene and nitrogen-doped carbon, which is applied in the field of electrochemistry, can solve the problems that are not involved in the research of supercapacitors, are not suitable for large-scale promotion and application, and fail to reduce the stacking of graphene, so as to achieve the improvement of composite materials. , not easy to overlap, shorten the effect of the distance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Disperse 3g of natural flake graphite in 70ml of concentrated sulfuric acid with a mass fraction of 98%, add 0.1g of sodium nitrate to cool down in an ice bath, then add 9g of potassium permanganate, keep the temperature below 20°C, and use 300- Stir the reaction at a rate of 500rpm for 1.5 hours; then place the reactant in a hot water bath at 38-40°C, and stir the reaction at a rate of 300-500rpm for 30min; Add distilled water and let it stand for at least 2 hours. After the solution is layered, discard the supernatant and centrifuge (13000rpm) for 10min. Take the dark solution obtained by centrifugation and ultrasonicate (20kHz) for 10min; then centrifuge again (4000rpm) for 10min. The upper yellow transparent liquid obtained afterward is graphene oxide;
[0032] (2) Adjust the concentration of graphene oxide obtained in step (1) to 0.5 mg / ml, take 100 ml of graphene oxide in a beaker, add 1.5 g of potassium permanganate, stir and react at a rate of 300-500 rpm fo...
Embodiment 2
[0045] Embodiment 2 hydrothermal reaction temperature influence test on composite material
[0046] Taking the hydrothermal reaction temperature (120°C, 130°C, 140°C) in step (5) of Example 1 as a variable, the influence of different ratios of graphene and carbon nanotubes on the performance of composite materials was tested. The tests were divided into the following groups:
[0047] Group 1: The mass ratio of porous graphene to carbon nanotubes is 5:1;
[0048] Group 2: The mass ratio of porous graphene to carbon nanotubes is 10:1;
[0049] Group 3: The mass ratio of porous graphene to carbon nanotubes is 15:1;
[0050] Group 4: without adding carbon nanotubes;
[0051] Group 5: Graphene does not create holes;
[0052] In groups 1-4, except that the ratio of porous graphene to carbon nanotubes is added in step (3), and the hydrothermal reaction temperature in step (5) is different, the rest of the steps are the same as in Example 1;
[0053] Group 5 is the same as that of...
Embodiment 3
[0058] Example 3 Different calcination temperatures affect the performance of composite materials
[0059] Taking the calcination reaction temperature (300°C, 350°C, 400°C) in step (6) of Example 1 as a variable, the influence of different ratios of graphene and carbon nanotubes on the performance of composite materials was tested. The tests were divided into:
[0060] Group 1: The mass ratio of porous graphene to carbon nanotubes is 5:1;
[0061] Group 2: The mass ratio of porous graphene to carbon nanotubes is 10:1;
[0062] Group 3: The mass ratio of porous graphene to carbon nanotubes is 15:1;
[0063] Group 4: without adding carbon nanotubes;
[0064] Group 5: Graphene does not create holes;
[0065] In groups 1-4, except that the ratio of porous graphene to carbon nanotubes is added in step (3), and the calcination reaction temperature in step (6) is different, the rest of the steps are the same as in Example 1;
[0066] Group 5 is the same as that of Example 1 excep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com