Application of biphenyl compound in preparing medicines for treating oxidative stress induced diseases
A technology of oxidative stress and compounds, applied in the field of medicine, can solve the problems of various and complex components, achieve the effect of inhibiting cell damage and apoptosis, and increasing the level of GSH
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation and structure confirmation of 3,3',4,4'-tetrahydroxybiphenyl
[0055] The method for obtaining 3,3',4,4'-tetrahydroxybiphenyl is as follows: extract the aerial part of Cinnamomum camphora with 95% ethanol to obtain an ethanol extract, and then sequentially extract with petroleum ether and ethyl acetate. The ethyl acetate fraction was eluted with petroleum ether-ethyl acetate gradient to obtain 19 fractions (A-S). Part N was separated by Sephadex LH-20 chromatographic column to obtain 3,3',4,4'-tetrahydroxybiphenyl.
[0056] Using the prepared 3,3',4,4'-tetrahydroxybiphenyl as raw material, slowly add triethylamine (4mmol) to 3,3',4,4 '-Tetrahydroxybiphenyl (1mmol), carbon tetrabromide (4.4mmol) and dibenzyl phosphite (5mmol) in anhydrous acetonitrile solution, followed by stirring at room temperature for 2 hours, after the reaction was completed, the solvent was evaporated under reduced pressure, The resultant was dissolved in ethyl acetate, was...
Embodiment 2
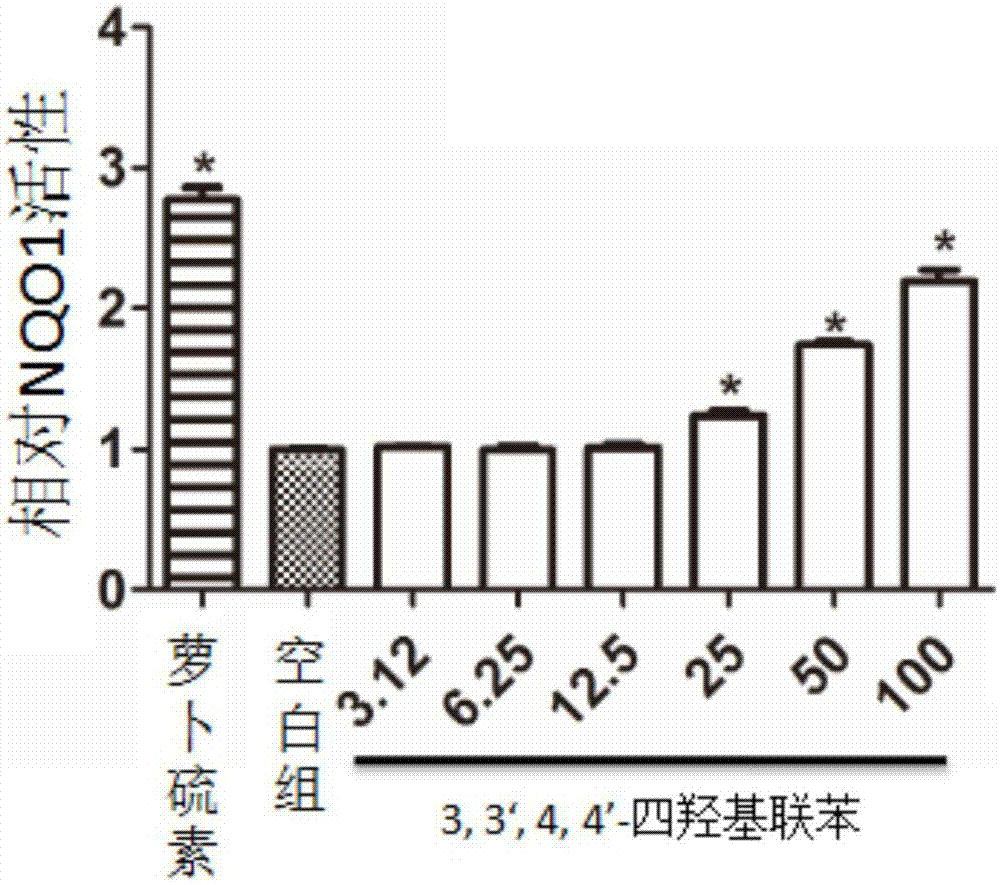
[0060] Example 2: Evaluation of NQO1-inducing activity of 3,3',4,4'-tetrahydroxybiphenyl
[0061] (1) Culture of mouse hepatocarcinoma hepa 1c1c cell line
[0062] The mouse hepatocarcinoma hepa 1c1c cell line was purchased from the American Type Culture Collection (ATCC), using MEM medium containing 10% fetal bovine serum (FBS), placed at 37°C, 5% CO 2 cultured in an incubator.
[0063] (2) NQO-inducing activity test
[0064] Hepa 1c1c cells were seeded on a 96-well plate, and different concentrations of 3,3',4,4'-tetrahydroxybiphenyl (confirmed in Example 1) were added after the cells adhered to the wall, and treated for 24 hours, using 0.8% digitonin The solution was used to lyse the cells, and the detection solution (1.0mL 0.5M Tris-hydrochloric acid (Tris-HCl), 15mg bovine serum albumin, 6mg MTT, 150μL Tween-20, 150μL 150mM D-glucose-6-phosphate, 15 μL 7.5 mM flavin adenine dinucleotide, 27 μL 50 mM nicotinamide adenine dinucleotide phosphate, 20 μL 50 mM menadione), l...
Embodiment 3
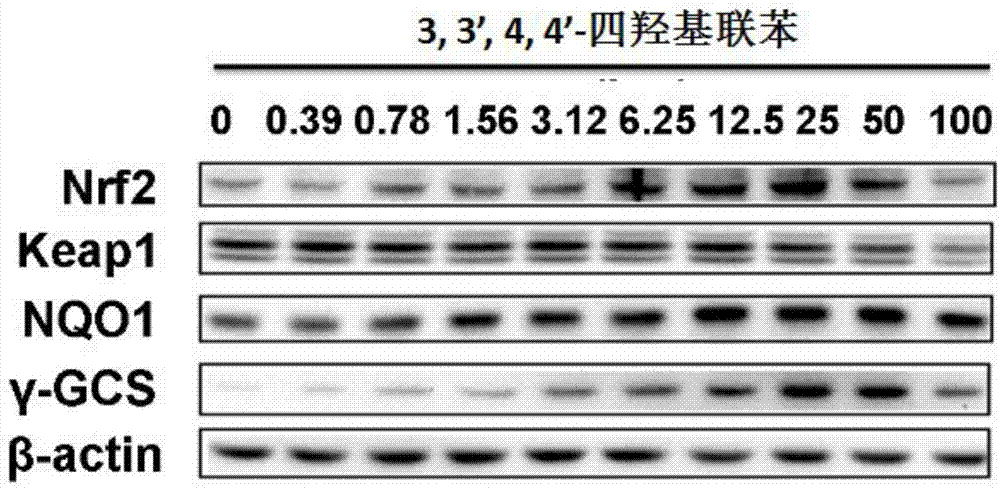
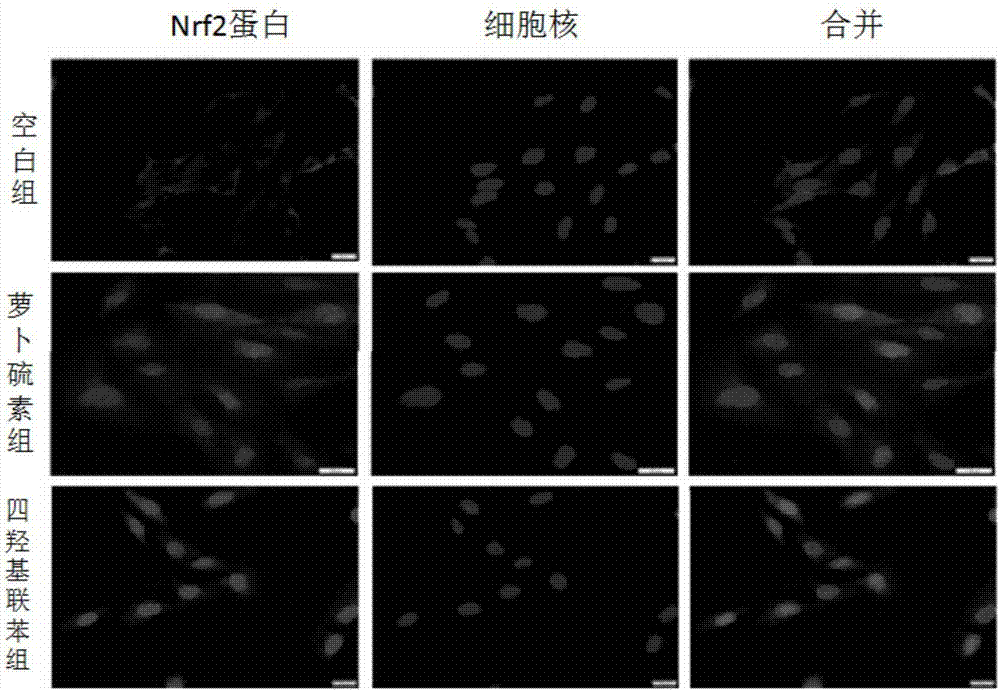
[0066] Example 3: 3,3',4,4'-Tetrahydroxybiphenyl can up-regulate Nrf2, γGCS and NQO1 protein levels
[0067] Method: Western blot analysis (Western blot) to detect changes in protein levels in cells
[0068] Beas-2B cells were inoculated in a 35 mm diameter petri dish, cultured until the density reached 70%-80%, and then treated with different concentrations of compounds to be tested for 16 h, washed twice with PBS, added cell lysate (50 μg / ml aprotinin , 0.5mM phenylmethylsulfonyl fluoride, 1mM sodium orthovanadate, 10mM sodium fluoride, 10mMβ-glycerol phosphate), the protein was collected and the protein concentration was determined by Bradford method. Each sample protein (100 μg) was loaded on the sample, and the protein components were separated by SDS-PAGE, and the protein bands were transferred to the nitrocellulose membrane by electrotransfer method. After the film was sealed with 5% skimmed milk powder solution prepared in TBS at room temperature for 1 hour, it was in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com