Serum-free culture medium for hybridoma cell
A technology of serum-free medium and hybridoma cells, which is applied in the direction of cell culture medium, cell culture active agent, culture process, etc. It can solve the impact of product quality, the difficulty of separation and purification of target antibodies, and the complicated production process of hybridoma culture, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
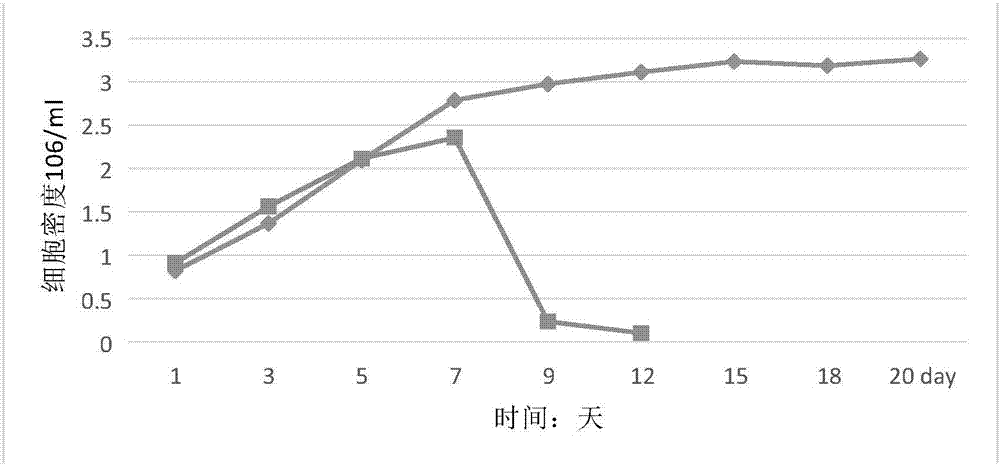
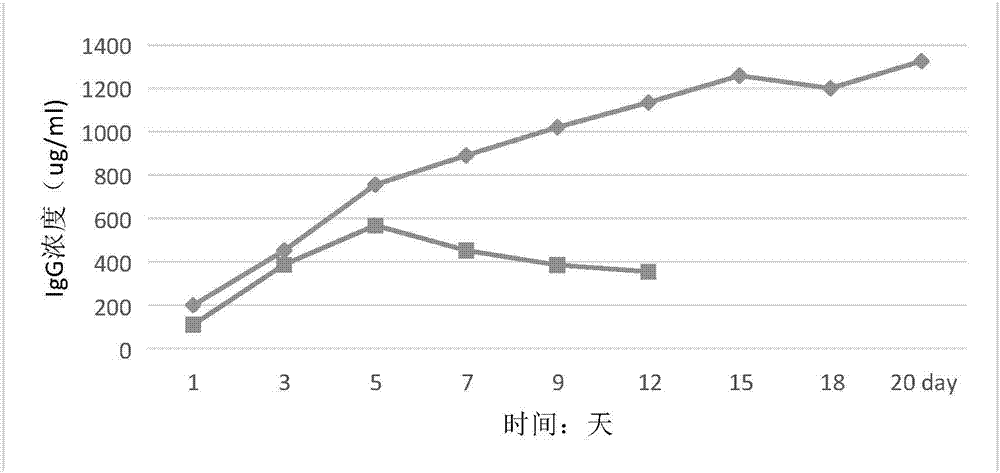
[0017] A serum-free medium for hybridoma cells, including raw materials at the following concentrations: alanine 50mg / L, arginine 40mg / L, aspartic acid 30mg / L, asparagine 50mg / L, cystine 40mg / L, cysteine 70mg / L, glutamine 600mg / L, glutamic acid 20mg / L, glycine 30mg / L, histidine 50mg / L, hydroxyproline 70mg / L, isoleucine 80mg / L, leucine 30mg / L, lysine 90mg / L, methionine 60mg / L, phenylalanine 50mg / L, proline 40mg / L, serine 50mg / L, threonine 40mg / L, color Amino acid 30mg / L, tyrosine 50mg / L, valine 30mg / L, calcium chloride 40mg / L, magnesium sulfate 35mg / L, potassium chloride 700mg / L, potassium dihydrogen phosphate 60mg / L, hydrogen phosphate Disodium 40mg / L, sodium bicarbonate 1g / L, sodium hydroxide 3g / L, ferrous sulfate heptahydrate 40mg / L, magnesium chloride hexahydrate 70mg / L, copper sulfate 30mg / L, sodium pyruvate 40mg / L, di Manganese chloride 50mg / L, sodium selenite 40mg / L, ammonium molybdate 5mg / L, sodium butyrate 70mg / L, sodium succinate 30mg / L, iron citrate 20mg / L, citr...
Embodiment 2
[0019]A serum-free medium for hybridoma cells, comprising raw materials at the following concentrations: alanine 10mg / L, arginine 10mg / L, aspartic acid 10mg / L, asparagine 10mg / L, cystine 10mg / L, cysteine 10mg / L, glutamine 100mg / L, glutamic acid 10mg / L, glycine 10mg / L, histidine 10mg / L, hydroxyproline 10mg / L, isoleucine 10mg / L, leucine 10mg / L, lysine 10mg / L, methionine 10mg / L, phenylalanine 10mg / L, proline 10mg / L, serine 10mg / L, threonine 10mg / L, color Amino acid 10mg / L, tyrosine 10mg / L, valine 10mg / L, calcium chloride 10mg / L, magnesium sulfate 10mg / L, potassium chloride 10mg / L, potassium dihydrogen phosphate 10mg / L, hydrogen phosphate Disodium 10mg / L, Sodium Bicarbonate 0.1g / L, Sodium Hydroxide 0.1g / L, Ferrous Sulfate Heptahydrate 10mg / L, Magnesium Chloride Hexahydrate 10mg / L, Copper Sulfate 10mg / L, Sodium Pyruvate 10mg / L , manganese dichloride 10mg / L, sodium selenite 10mg / L, ammonium molybdate 0.001mg / L, sodium butyrate 10mg / L, sodium succinate 10mg / L, iron citrate 10mg / ...
Embodiment 3
[0021] A serum-free medium for hybridoma cells, including raw materials at the following concentrations: alanine 100mg / L, arginine 1100mg / L, aspartic acid 100mg / L, asparagine 100mg / L, cystine 100mg / L, cysteine 100mg / L, glutamine 1000mg / L, glutamic acid 100mg / L, glycine 100mg / L, histidine 100mg / L, hydroxyproline 100mg / L, isoleucine 100mg / L, leucine 100mg / L, lysine 100mg / L, methionine 100mg / L, phenylalanine 1100mg / L, proline 100mg / L, serine 100mg / L, threonine 100mg / L, color Amino acid 100mg / L, tyrosine 100mg / L, valine 100mg / L, calcium chloride 100mg / L, magnesium sulfate 100mg / L, potassium chloride 1000mg / L, potassium dihydrogen phosphate 100mg / L, hydrogen phosphate Disodium 100mg / L, sodium bicarbonate 5g / L, sodium hydroxide 5g / L, ferrous sulfate heptahydrate 100mg / L, magnesium chloride hexahydrate 100mg / L, copper sulfate 100mg / L, sodium pyruvate 100mg / L, di Manganese chloride 100mg / L, sodium selenite 100mg / L, ammonium molybdate 100mg / L, sodium butyrate 100mg / L, sodium succi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com