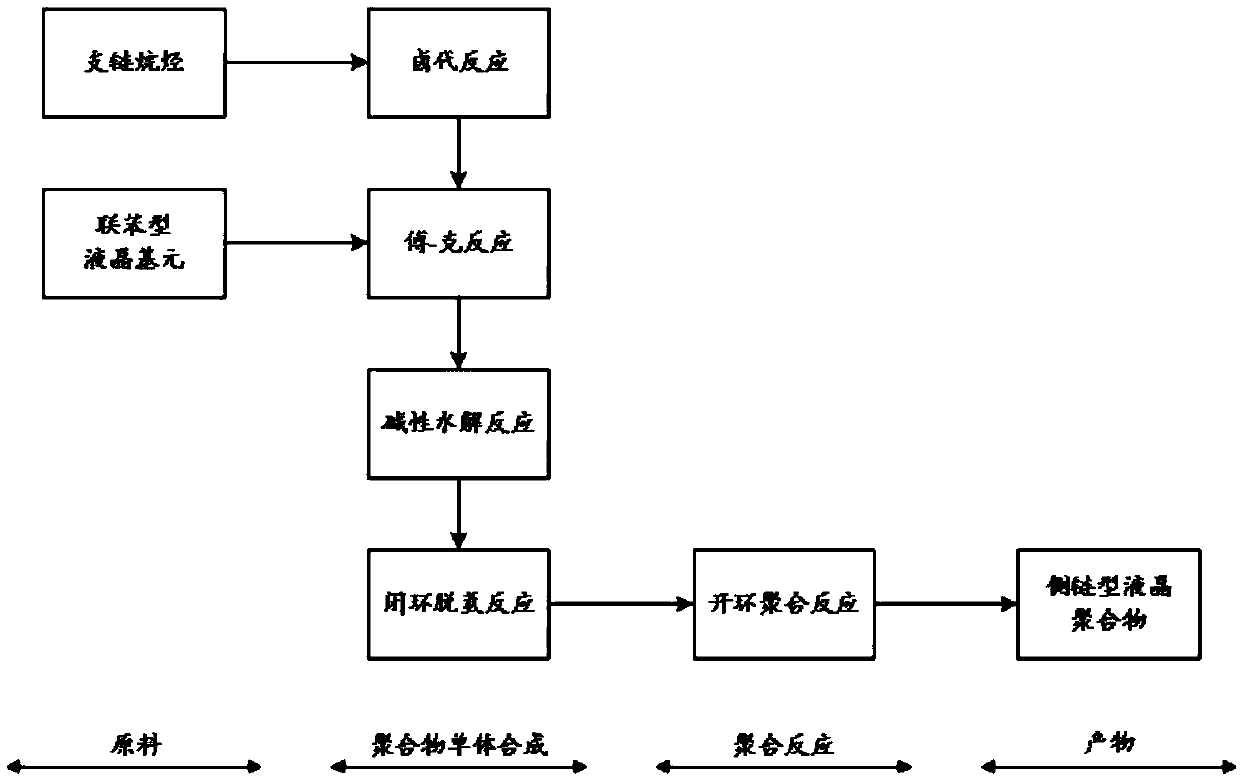
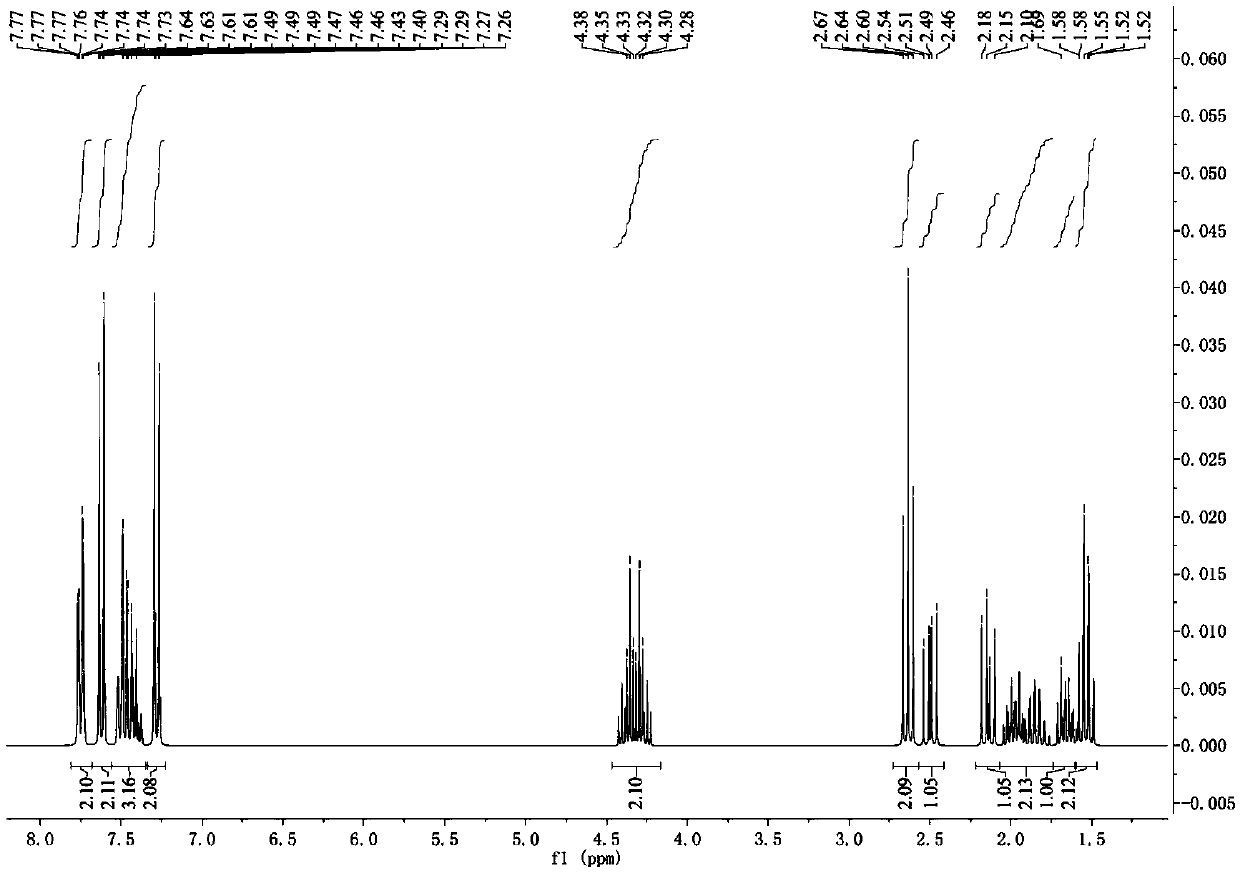
Lactone aromatic derivative containing liquid crystal element, preparation method thereof, side chain type liquid crystal polymer and preparation method and application thereof
A liquid crystal cell and derivative technology, applied in liquid crystal materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of expensive precious metal catalysts, incomplete hydrosilylation reaction, wide molecular weight distribution, etc., and achieve cheap catalysts. Easy to obtain, good phase change characteristics, narrow molecular weight distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] (1) Synthesis of 3-(2-chloroethyl)-1,5-dichloropentane
[0091]
[0092] Add 20 g of 3-ethyl-pentane to a three-necked round-bottom flask connected with a stirrer and a condenser, vacuumize and replace with nitrogen 5 times, and add 200 g of chloroform and 120 g of thionyl chloride under the protection of a nitrogen atmosphere. Under the light of 500 lux, the water bath was heated to 70° C. for reflux reaction for 2 h. The flask was transferred to an ice-water bath to terminate the reaction, purged with nitrogen, and the tail gas was absorbed with lye. The solvent chloroform and excess thionyl chloride were recovered by distillation, the product was washed with saturated sodium bicarbonate solution until neutral, the organic phase was dried, filtered, and purified by rectification under reduced pressure to collect 3-(2-chloroethyl)-1 , 13.84 g of 5-dichloropentane.
[0093] (2) Alkylation reaction to prepare 4-(5-chloro-3-(2-chloroethyl)pentyl)-1,1’-biphenyl
[00...
Embodiment 2~7
[0103] The preparation method is the same as in Example 1, the main parameter values of the reaction process are shown in Table 1, and the raw materials and product structure are shown in Table 2:
[0104] Table 1
[0105]
[0106]
[0107] Table 2
[0108]
Embodiment 8
[0110] Using Example 1 to prepare the ring-opening polymerization of lactone derivatives to prepare liquid crystal polymers:
[0111]
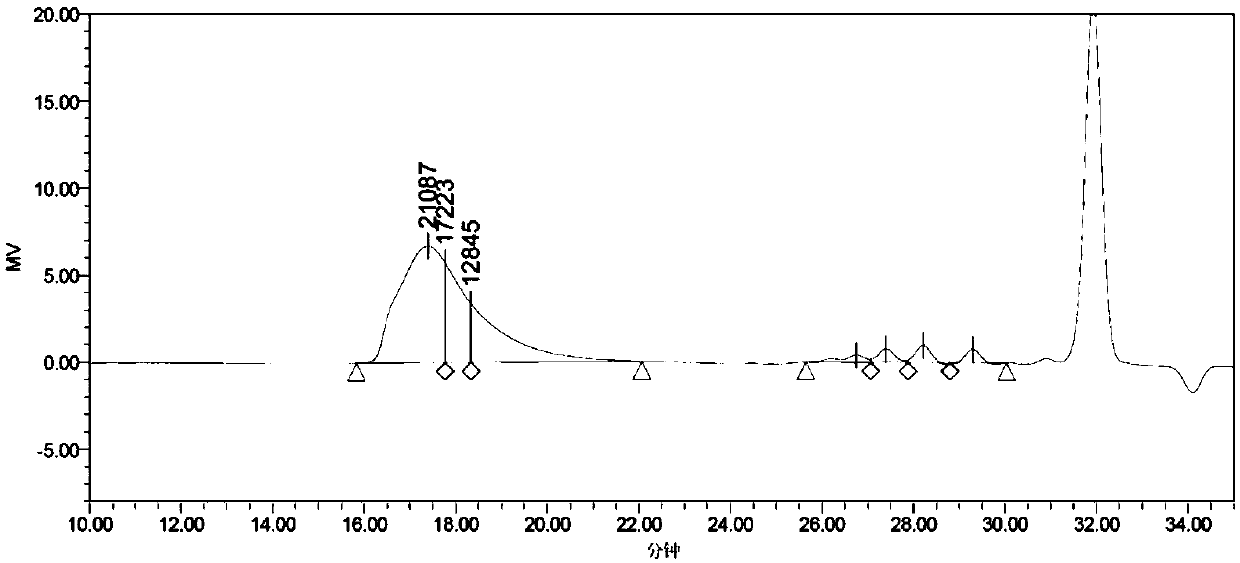
[0112] In the reactor connected with nitrogen pipeline, add 28g (0.1mol) of 3-biphenylethyl-valerolactone, toluene 150g, pentafluorophenol 50g, initiator n-butanol 0.106g, catalyst trifluoromethanesulfonic acid 0.0056g (200ppm), replace the air with nitrogen to remove the air in the kettle, fill it with nitrogen until the pressure is 0.1Mpa(G), start stirring and fully dissolve, set the speed at 300rpm, gradually raise the reaction temperature to 100°C, stop the reaction after 4 hours of reaction, Lower the temperature of the system to 60°C, collect the product into a flask, add 100g of ethanol, place the flask in a cold water bath at 10°C to fully precipitate the polymer, filter and wash it, and dry it under vacuum at 60°C to obtain 26.37g of liquid crystal polymer powder. Sample GPC test Mn is 17223 (see image 3 ), the PDI is 1.14, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com