Preparation method of nickel nanowire array electrode and application of nickel nanowire array electrode as electrochemical oxygen evolution active material
A silicon nanowire array and array electrode technology, which is applied in the field of electrochemical oxygen evolution, can solve the problems of low catalytic activity and insufficient durability, and achieve the effects of simple preparation and modification methods, good OER activity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
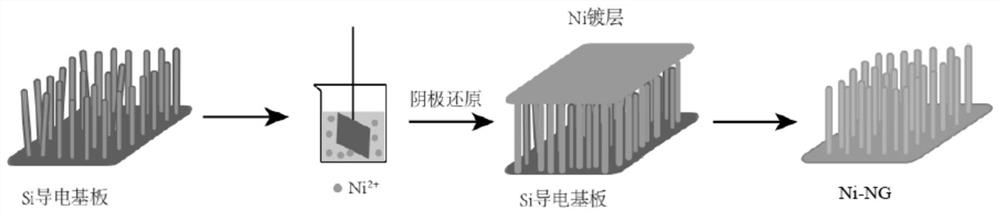
[0025] Embodiment 1: the preparation of silicon template
[0026] The silicon nanowire array structure prepared by deep reactive ion etching (DRIE) was used as a template. In this work, a silicon wafer was used as the original material, and its detailed parameters are listed in Table 1:
[0027] Table 1 Detailed parameters of silicon wafer
[0028]
[0029] The experiment used a high-density plasma etching machine (IPC-multiplex) produced by Surface Technology Systems (STS) in the UK. The silicon wafer will be transferred to a chamber that maintains highly anisotropic etching. This etching process Based on successive passivation and etch cycles. In this experiment, 75 continuous etching cycles were used to obtain a silicon nanowire array structure with a depth of 20 μm. In each cycle, octafluorocyclobutane (C 4 f 8 ) gas for passivation treatment, the passivation time is 11s, and the flow rate is 85sccm; subsequently, in sulfur hexafluoride (SF 6 ) and O 2 Etching in ...
Embodiment 2
[0030] Embodiment 2: the preparation of Ni-NG electrode
[0031] In order to obtain a nickel nanowire array structure with a high surface area, nickel was deposited on the silicon template by electroplating. First, put the prepared silicon template into the platform groove made of polymethyl methacrylate (PMMA), then fix the silicon template with adhesive tape and connect it with copper wire as the cathode material; polypropylene (pp ) mesh-coated high-purity (99.99%) electrolytic nickel sheet is the anode material; the electrolyte is prepared with analytically pure grade chemical reagents and deionized water, and the composition of the electrolyte is nickel sulfamate (Ni(NH 2 SO 3 ) 2 4H 2 O), boric acid (H 3 BO 3 ) and sodium lauryl sulfate (NaC 12 h 25 SO 4 ), where Ni(NH 2 SO 3 ) 2 4H 2O provides nickel ions with a concentration of 120g / L, H 3 BO 3 The concentration is 30g / L, and 0.5g of NaC in powder form is added to each liter of electrolyte 12 h 25 SO 4...
Embodiment 3
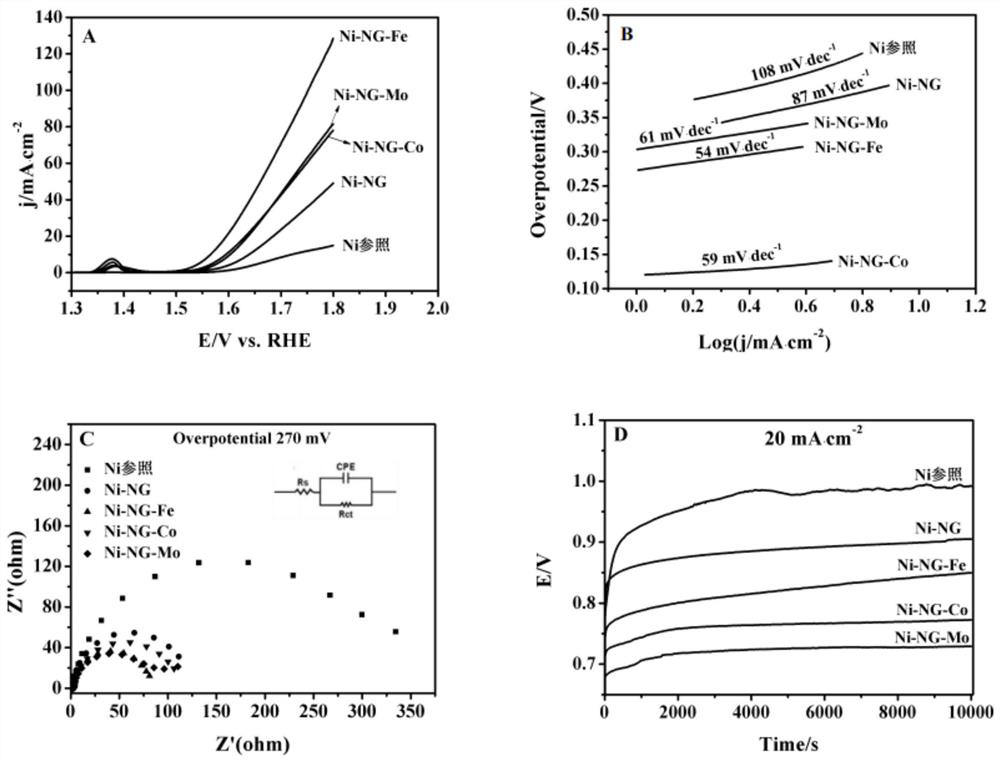
[0037] Embodiment 3: the modification of Ni-NG electrode
[0038] In the electrocatalytic oxidation process, the electrocatalytic OER activity of Ni-NG is not high enough, and the introduction of transition metal atoms to modify the Ni-NG interface can significantly optimize its OER catalytic activity and durability. First, use ultrapure water to prepare concentrations of 0.5g / 100mLFeCl 3 , 0.5g / 100mL CoCl 2 , 0.5g / 100mL molybdic acid solution, take three Ni-NG electrodes with an area of 1cm×1cm and soak them in the prepared FeCl 3 、CoCl 2 , Molybdenum acid solution, soaking time is 30min, rinse gently with deionized water for 2-3min after soaking, then place the sample in an oven for drying treatment, and finally use a tube furnace to dry the obtained material in an Ar atmosphere Then, the temperature was increased from room temperature to 250 °C at a rate of 5 °C / min for calcination and maintained for 2 h, and finally Ni-NG-Fe, Ni-NG-Co, Ni-NG-Mo electrode materials wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com