A near-infrared fluorescent probe for detecting cyp 1A1 enzyme
A CYP1A1, fluorescent probe technology, applied in the field of near-infrared fluorescent probes, can solve the problems of organism interference and low selectivity, and achieve the effect of high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The fluorescent probe of the present invention 2-((6-((4-(2-bromoethoxy)benzyl)oxy)-2,3-dihydro-1H-xanthin-4-yl)methylene) Malononitrile (BrDXMM) is prepared by the following method:
[0031] 1 mmol of HDXMM (HDXMM of the present invention was prepared by the synthetic method disclosed in the following document: Sensors & Actuators: B. Chemical 273 (2018) 167-175), 2 mmol of potassium carbonate and 1.2 mmol of 4-(2-bromoethoxy) ) benzyl bromide was placed in a 25mL two-necked flask, then 8mL of N,N-dimethylformamide was added to the two-necked flask, the reaction mass was heated to 90°C under nitrogen protection, and the reaction was stirred for 1 hour; after the reaction Cooled to room temperature, the reaction solution was added to cold water, stirred vigorously, and a large amount of dark red solids were precipitated, filtered, and the filter cake was separated by column chromatography (developing solvent: ethyl acetate: petroleum ether=5:1, v:v ) to obtain a dark r...
Embodiment 2
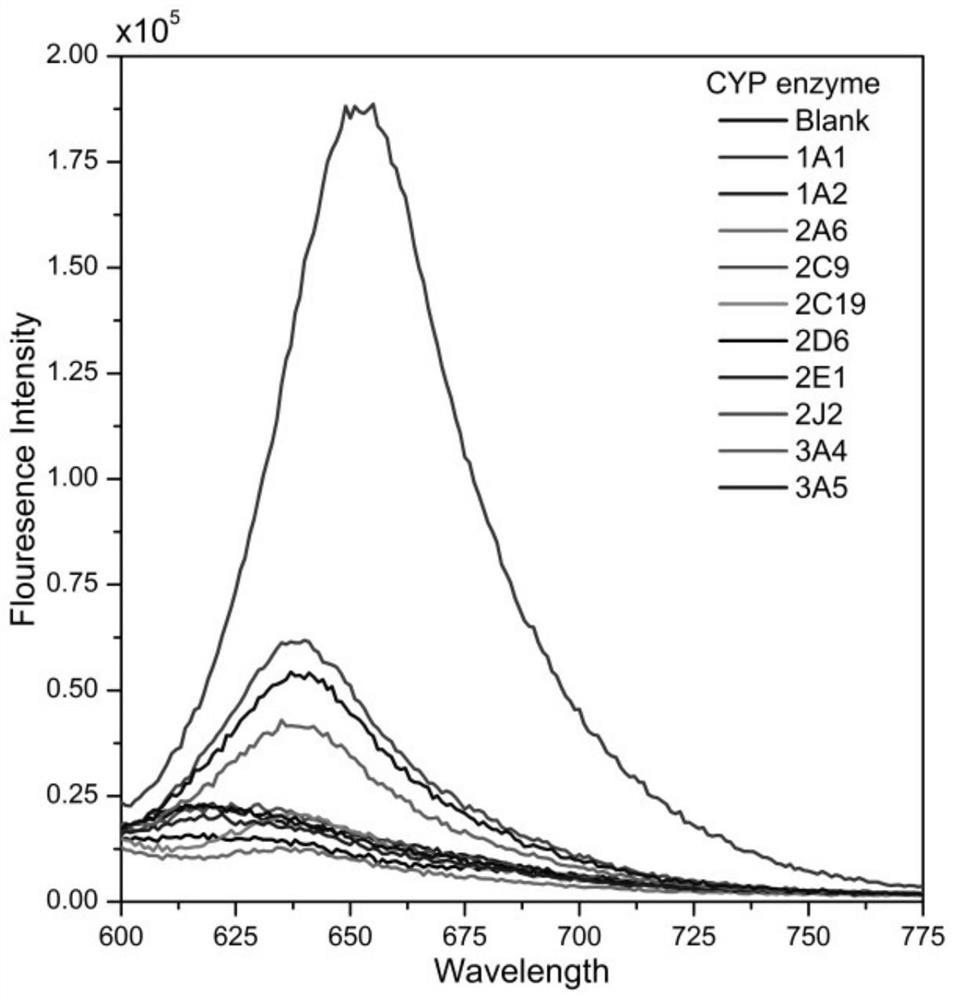
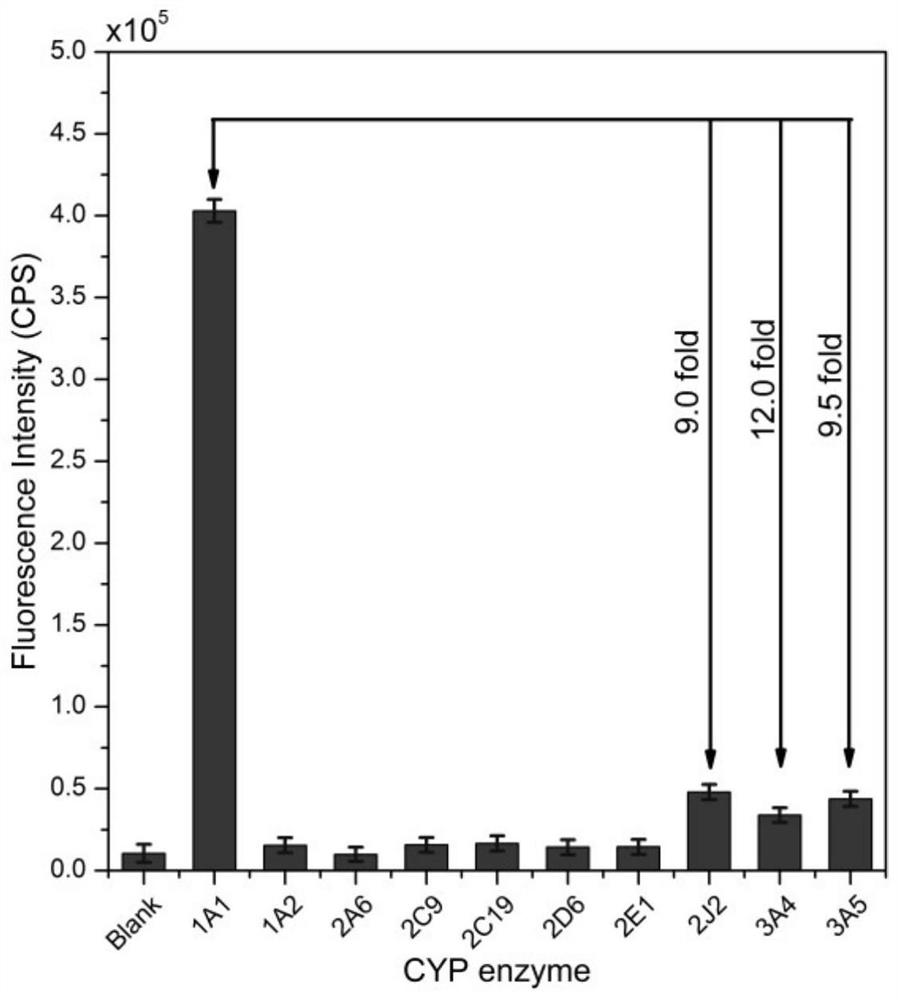
[0032] Example 2: Determination of the selectivity of fluorescent probe BrDXMM for each isoform of CYP450
[0033]Preparation of phosphate buffer solution (PBS): Weigh sodium dihydrogen phosphate dihydrate (0.7410g), dodecahydrate disodium hydrogen phosphate (7.2495g) and magnesium chloride hexahydrate (0.2030g) with an analytical balance and place them in a 100mL beaker , add double distilled water (200 mL in total) to the beaker in 4 times to fully dissolve, let stand, transfer to a 250 mL volumetric flask, add double distilled water to make the volume, fully shake evenly, and then use a pH meter to check the pH of the buffer . Prepared to obtain PBS buffer (0.1M, pH 7.4, c(MgCl) 2 ) = 4.0 mM), stored at 4°C.
[0034] Preparation of reaction substrate solution: Weigh BrDXMM and dissolve it in dimethyl sulfoxide to prepare a mother solution with a concentration of 5 mM. Take 40 μL of the stock solution and add it to 4.96 mL of PBS buffer to prepare a 40 μM reaction substra...
Embodiment 3
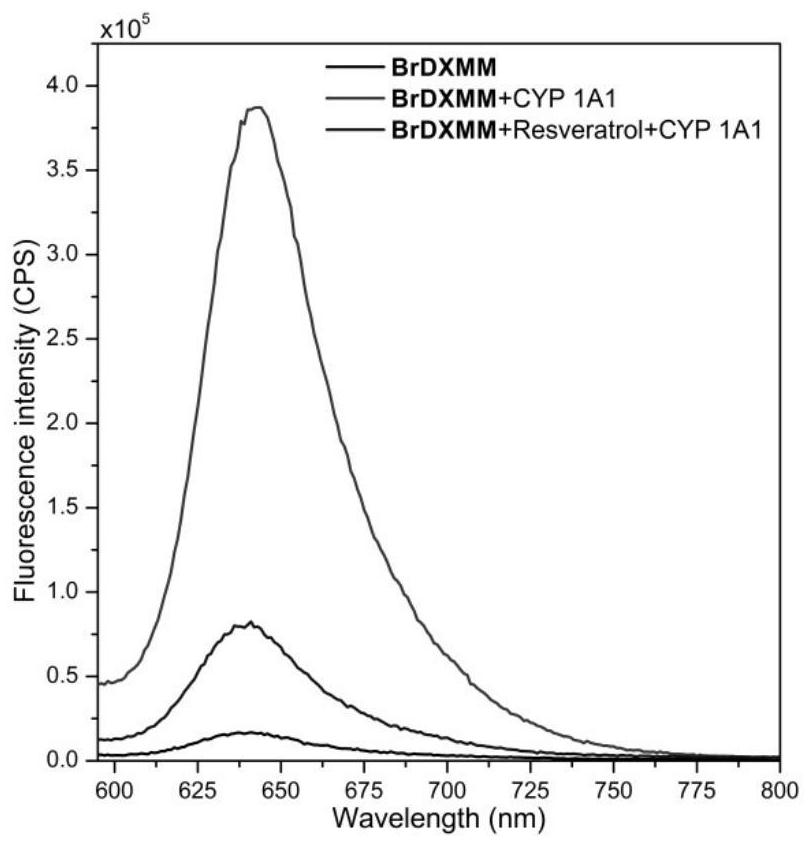
[0037] Example 3: Chemical inhibition experiment of fluorescent probe BrDXMM
[0038] 100 μL of the above-mentioned glucose-6-phosphate solution, 50 μL of β-nicotinamide adenosine phosphate solution, 5 μL of glucose-6-phosphate dehydrogenase solution, and 5 μL of CYP 1A1 ( Final concentration 50nM), resveratrol (final concentration 25μM, CYP 1A1 specific inhibitor), after mixing, add 10μL, 40μM BrDXMM (final concentration 10μM), and finally diluted with PBS buffer to the corresponding concentration. The above mixture was then mixed and incubated in a constant temperature water bath at 37°C for 60 minutes. At the end of the incubation, dimethyl sulfoxide (200 μL) was added to the reaction system to stop the reaction and mix well. The mixture was cryogenically centrifuged (13,300 × g, 4 °C) for 20 min, and the supernatant was taken for fluorescence analysis, via image 3 It can be seen that the fluorescent probe BrDXMM of the present invention indeed produces a fluorescent res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com