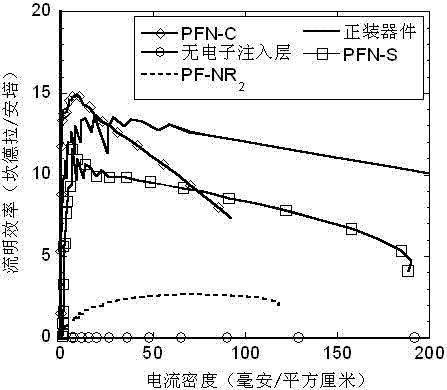
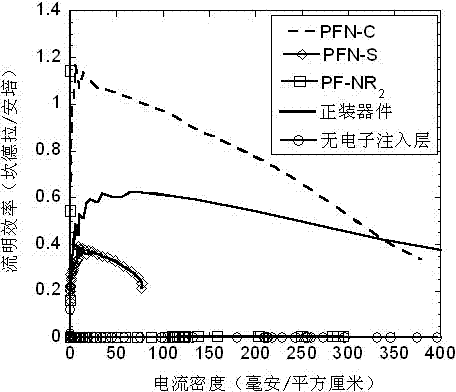
Application of cross-linkable conjugated polymer materials in flip-chip organic optoelectronic devices
A technology of conjugated polymers and optoelectronic devices, which is applied in the direction of electrical solid devices, electrical components, semiconductor devices, etc., can solve the problems of insufficient processing methods, solvent erosion, and unsatisfactory charge transmission, etc., and achieve good thermal stability and electrochemical stability. Stability, simple device preparation process, and the effect of overcoming interfacial miscibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Crosslinkable conjugated polymer poly{2,7-[9,9'-bis(3-ethyl-3-(6-hexyl)methyl ether-oxetane)fluorene]-co-2 , Preparation of 7-[9,9'-bis(6-N,N-diethylamino-hexyl)fluorene]} (referred to as PFN-C)
[0050] The synthetic route is as follows:
[0051]
[0052] (1) Monomer 1 [2,7-dibromo-9,9'-bis(3-ethyl-3-(6-hexyl)methyl ether-oxetane)fluorene] was obtained according to the literature [ J. Polym. Sci. A Polym. Chem., 2007, 3, 388] prepared by the disclosed method, monomer 3 [2,7-bis(trimethylene borate)-9,9'-bis(6- N,N-Diethylamino-hexyl)fluorene] was prepared according to the method disclosed in "Advanced Materials" [Adv. Mater., 2011, 23, 1665].
[0053] (2) Poly{2,7-[9,9'-bis(3-ethyl-3-(6-hexyl)methyl ether-oxetane)fluorene]-co-2,7-[ Preparation of 9,9'-bis(6-N,N-diethylamino-hexyl)fluorene]} (referred to as PFN-C)
[0054] Monomer 2,7-bis(trimethylene borate)-9,9'-bis(6-N,N-diethylamino-hexyl)fluorene (728 mg, 1 mmol), monomer 2,7 -Dibromo-9,9'-bis(3-ethyl-3...
Embodiment 2
[0057] Poly{2,7-[9,9'-bis(3-ethyl-3-(6-hexyl)methyl ether-oxetane)fluorene]-co-2,7-[9,9 Preparation of '-bis(6-N,N-diethylamino-hexyl)fluorene]} (abbreviated as PFN-S)
[0058] The synthetic route is as follows:
[0059]
[0060] (1) Preparation of monomer 1 [2,7-dibromo-9,9’-bis(6-formylphenoxyhexyl)fluorene]
[0061] Add the raw material 2,7-dibromo-9,9'-(6-bromohexyl)fluorene (32.5g, 50mmol) into the reaction flask, add 300ml N,N-dimethylformamide (DMF) to dissolve the raw material , then add p-Hydroxybenzaldehyde (15.3g, 125mmol) and potassium carbonate 2g, and heat to reflux under argon protection for 12 hours. After cooling, the reaction solution is poured into ice water, extracted with dichloromethane and concentrated to the concentrate The column was carried out to obtain 29.2 g of the product with a yield of 80%.
[0062] The NMR data of the product are as follows: 1 H NMR (300 MHz, CDCl 3 ), δ (ppm): 9.86 (s, 2H), 7.82-7.78 (d, 4H), 7.77-7.51 (d, 2H), 7.50-...
Embodiment 3
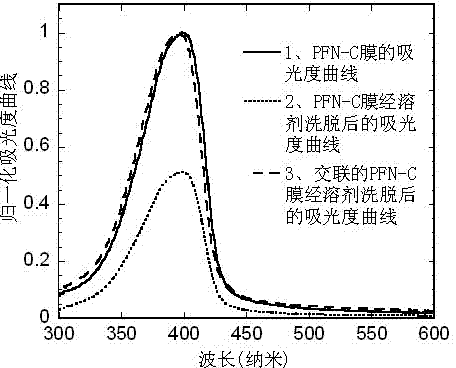
[0070] Taking the polymer PFN-C as an example to illustrate that this type of polymer has anti-solvent elution properties after cross-linking treatment
[0071] Dissolve the polymer PFN-C synthesized in Example 1 in p-xylene, and then add photoacid [2-(4-methoxystyryl)-4,6-bis(tri Chloromethyl)-1,3,5-triazine], wherein the role of photoacid is to provide hydrogen ions under ultraviolet light irradiation, so that cationic ring-opening polymerization of oxetane occurs. The solution was filtered with a 0.45 micron filter membrane, and spin-coated on a common glass slide to form a film with a thickness of about 20 nanometers. The absorbance of PFN-C after film formation was measured with a UV tester (HP 8453 spectrophotometer) produced by Hewlett-Packard Company, corresponding to figure 1 Curve 1 in . Afterwards, the PFN-C film is irradiated with ultraviolet light with a wavelength of 365 nm for 1 minute, and then heated on a heating plate to cause ring-opening polymerization...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com