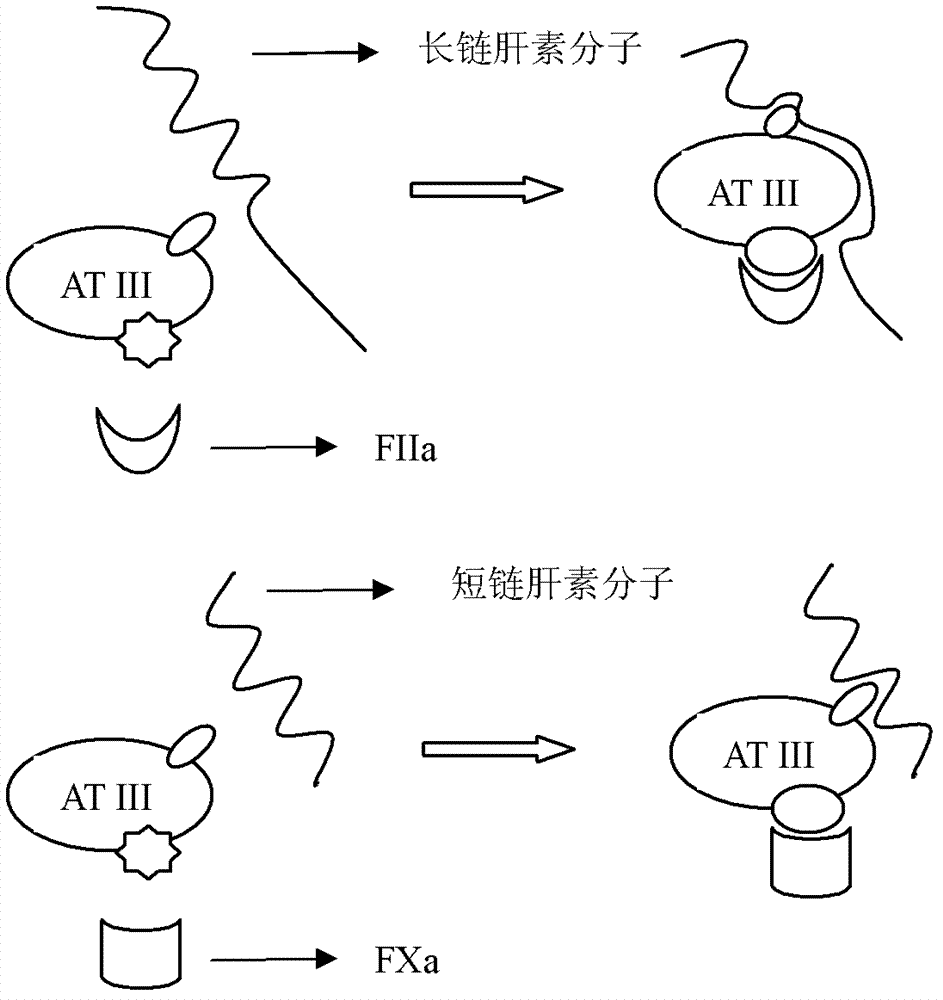
Straight-chain heparin modified biotype artificial blood vessel
A bio-based, artificial blood vessel technology, applied in the types of packaging items, anti-coagulation treatment, special packaging items, etc., can solve the problem that the regeneration and remodeling process is difficult to occur, and the surface properties cannot meet the needs of biomaterial compatibility and tissue regeneration. , Heparin activity is difficult to guarantee, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
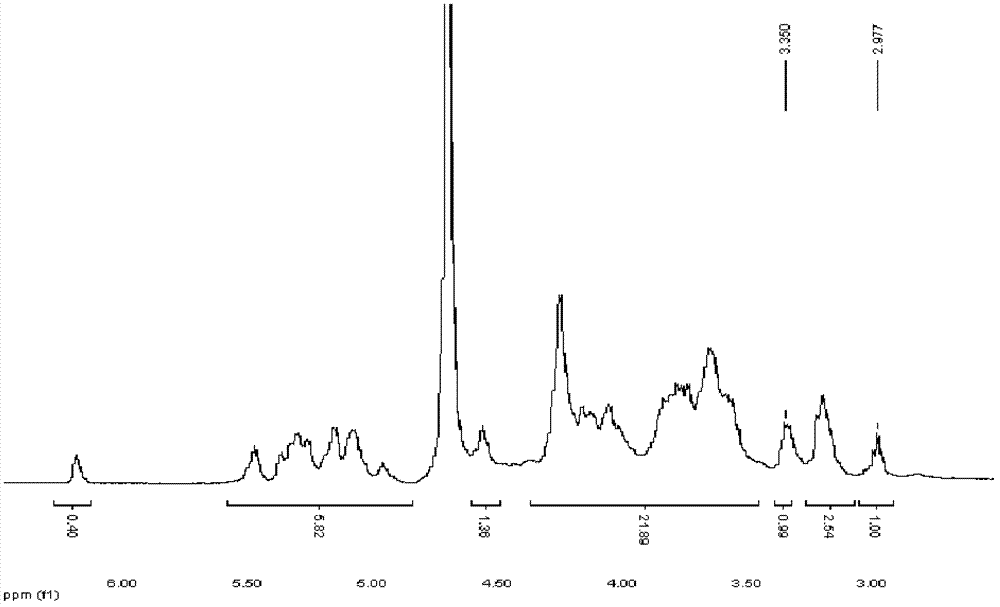
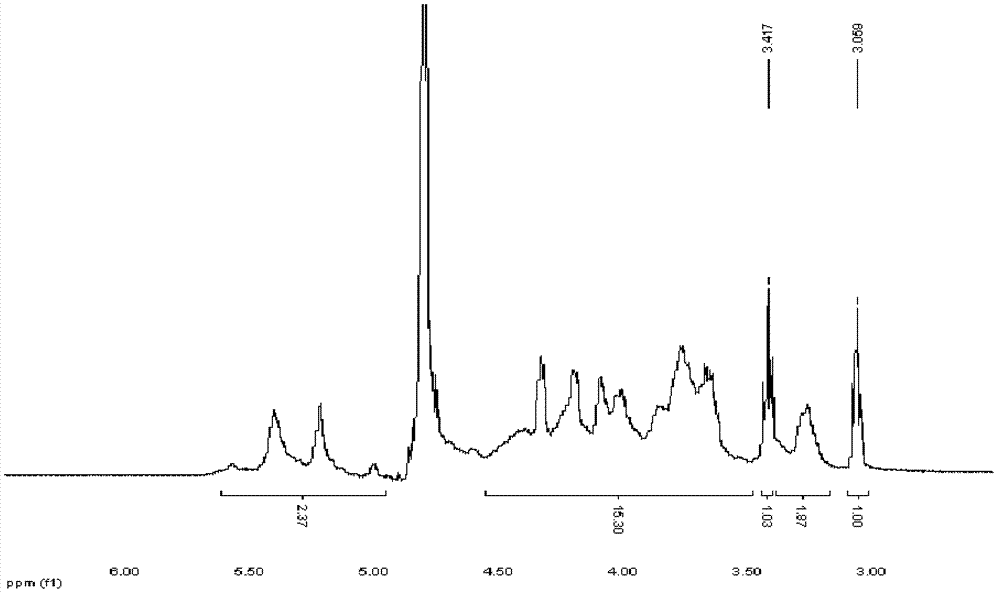
[0057] Example 1, Preparation of terminal cystamined heparin and terminal thiolated heparin
[0058] 1. Preparation of 3-5K and 5-10K heparin by enzymatic degradation
[0059] Heparin extracted from porcine small intestinal mucosa is degraded by type I heparanase for a certain period of time, and then ultrafiltered and dialyzed with an ultrafiltration membrane with strong molecular weight selectivity to obtain:
[0060] (1) Prepare 0.1M Tris-hydrochloric acid buffer solution, and adjust the pH between 7.00±0.02.
[0061] (2) Weigh 806.9 mg of heparin powder (white), and sterilize it by ultraviolet light for 30 minutes in a biological safety cabinet. This was added to 16.0 ml of Tris buffer filtered through a 220 micron pore filter. (take a sample and measure the pH value of the system with a pH meter, it is 7.08).
[0062] (3) Filter the bacteria with a filter membrane again. Add 100 μl of 100 units (sigma unit, about 1 / 600th international unit) heparinase-Tris buffer solu...
Embodiment 2
[0103] Example 2: Heparin compounded on the acellular matrix artificial blood vessel
[0104] After reducing the end of cystamined heparin, thiolated heparin is obtained, and then quantitatively detects the amount of amino groups on the inner surface of blood vessels, and then activates the inner surface of blood vessels through a linker with a thiol-amino bidirectional functional group, and then covalently immobilizes the heparin with a direct chemical bond. Chain-shaped heparin on the inner surface of the vascular material (see Figure 4 ).
[0105] 1. Detection of amino groups on blood vessels
[0106] Reaction principle: Molecules containing primary amine or hydrazide groups can react with
[0107] 2,4,6-trinitrobenzenesulfonate (TNBS) reacts to generate colored derivatives; the amino group content in the complex is linearly related to the absorption value of the orange-yellow product at 335nm. Therefore, the amino group content can be determined by measuring the absorb...
Embodiment 3
[0150] Example 3. Characterization of Anticoagulation Properties of Composite Artificial Blood Vessels
[0151] A few milliliters of blood is collected from the vein of healthy New Zealand rabbits, and 10% of the total volume of sodium citrate is added, which can have an anticoagulant effect in a short time. The collected fresh rabbit whole blood was centrifuged at 1500rpm in a common centrifuge for 15min, then the upper and middle liquids were drawn into another centrifuge tube, centrifuged again at 3000rpm for 10min, and after centrifugation, 75% of the supernatant was drawn and preserved, which was platelet-poor plasma (PPP ), and the remaining fluid is platelet-rich plasma (PRP). Among them, PPP is used for the detection of plasma recalcification time, prothrombin time, and activated partial thromboplastin time, and PRP is used for platelet adhesion test.
[0152] 1. Platelet adhesion assay (PAA)
[0153] 1) Each blood vessel sample (1cm 2 , refers to the double-sided s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com