Method for detecting trivalent chromic ions by using gamma-polyglutamic acid stabilized triiron tetraoxide nano grains
A technology of ferric oxide and polyglutamic acid, which is applied in the analysis of nuclear magnetic resonance, etc., can solve the problems of low-cost real-time detection, system influence, and bulky volume, and achieve good aqueous solution stability, short response time, and sensitivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Add γ-polyglutamic acid solution into the ferric chloride solution, stir and add sodium sulfite solution, stir for 30 minutes, add ammonia water in a 60°C water bath, and react for 1.5 hours. The upper layer solution was taken by centrifugation, and dialyzed to obtain a solution of iron ferric oxide nanoparticles stabilized by γ-polyglutamic acid. Wherein the mass ratio of ferric chloride to polyglutamic acid is 7:1, and the molar ratio of ferric chloride to sodium sulfite is 7:1.
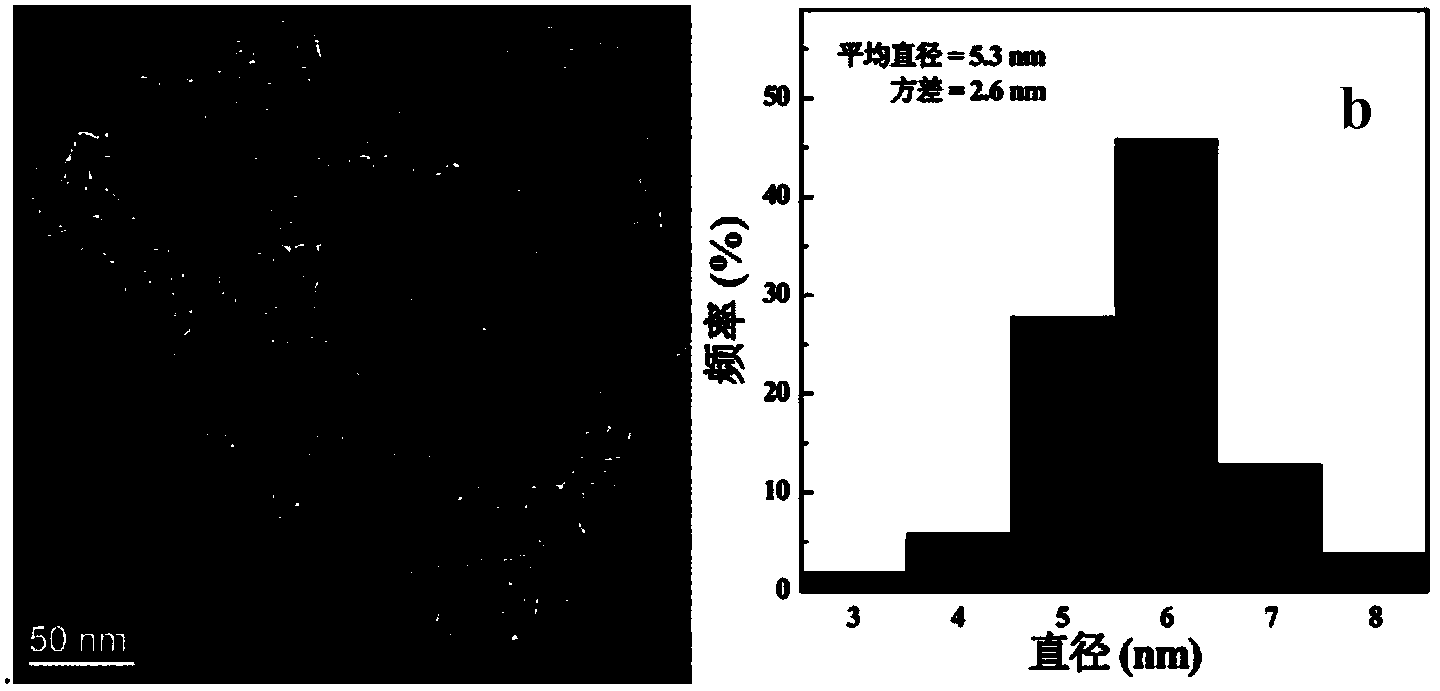
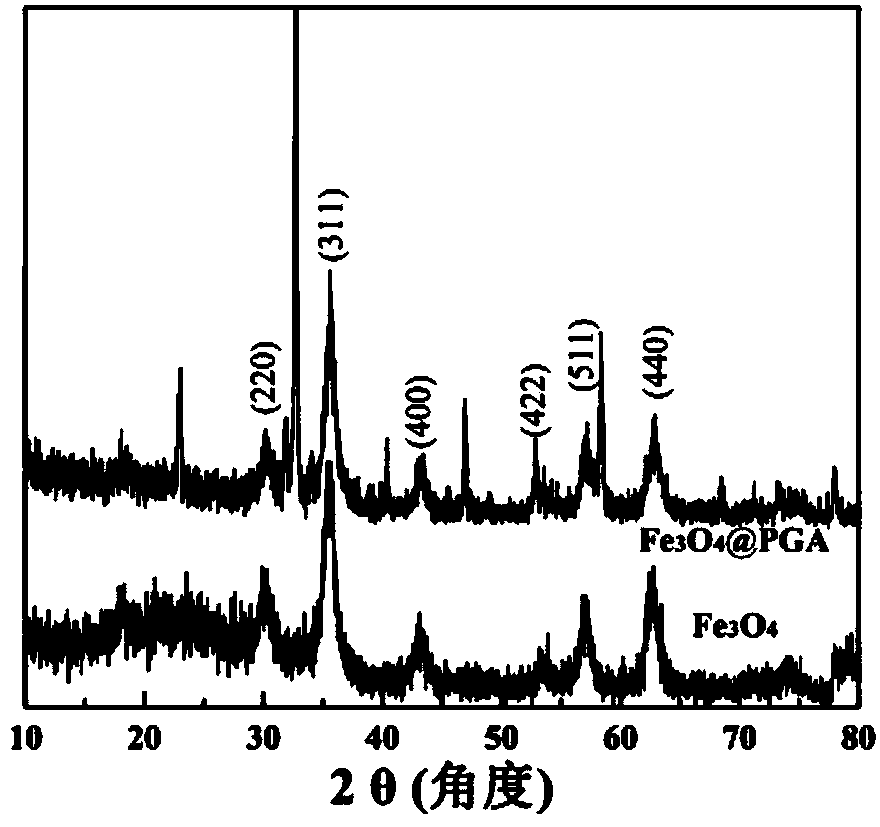
[0052] Observation by transmission electron microscope to the ferric oxide nanoparticles stabilized by gamma-polyglutamic acid prepared by the present invention shows that the ferric oxide nanoparticles formed are uniform, the average particle size is 5.3nm, and they are distributed in narrower range (see attached figure 1). The hydrodynamic particle size was measured by dynamic light scattering, and the result showed that the particle size was about 217.5nm. X-ray diffraction test proves...
Embodiment 2
[0054] Take 50 μL (concentration of 20 μM) of the γ-polyglutamic acid-stabilized iron ferric oxide nanoparticle solution prepared in Example 1, and add different volumes (0, 20, 40, 60, 80, 100 μL) of Cr 3+ Solution (concentration is 10nM), and set the volume to 1mL, place at room temperature for 10min, test the T of ferroferric oxide nanoparticles 2 The relaxation time changes, resulting in Cr 3+ Content and T 2 Quantitative relationship of relaxation times.
[0055] By adding different concentrations of Cr to the ferric oxide nanoparticles stabilized by gamma-polyglutamic acid prepared in the present invention 3+ The magnetic resonance detection results showed that the r 2 Value varies with Cr 3+ Concentration increases gradually (see appendix Figure 6 ), and its linear correlation coefficient R 2 = 0.9998. From Figure 6 It can be seen that Cr 3+ The addition of T to Fe3O4 nanoparticles 2 The relaxation time curve has a greater influence, while with the Cr 3+ co...
Embodiment 3
[0057] Get the gamma-polyglutamic acid stable iron ferric oxide nanoparticle solution 50 μ L (concentration is 20 μ M) that embodiment 1 prepares to be diluted to 1mL, test its T 2 relaxation time. In the same way, take a series of γ-polyglutamic acid-stabilized ferric oxide nanoparticle solutions, mix with 100 μL aqueous solution of chromium nitrate and potassium chloride, lead nitrate, cobalt chloride, nickel nitrate, calcium nitrate, magnesium chloride, Lithium sulfate and copper sulfate were mixed, diluted to 1mL solution, left at room temperature for 10min, and the T of ferric oxide nanoparticles was tested. 2 Relaxation time changes to verify the effect of Fe3O4 nanoparticles on Cr 3+ detection specificity. Wherein the concentration of potassium chloride, lead nitrate, cobalt chloride, nickel nitrate, calcium nitrate, magnesium chloride, lithium sulfate, copper sulfate is 10nM (final concentration is 1nM).
[0058] The magnetic resonance detection of ferric oxide solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relaxation rate | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com