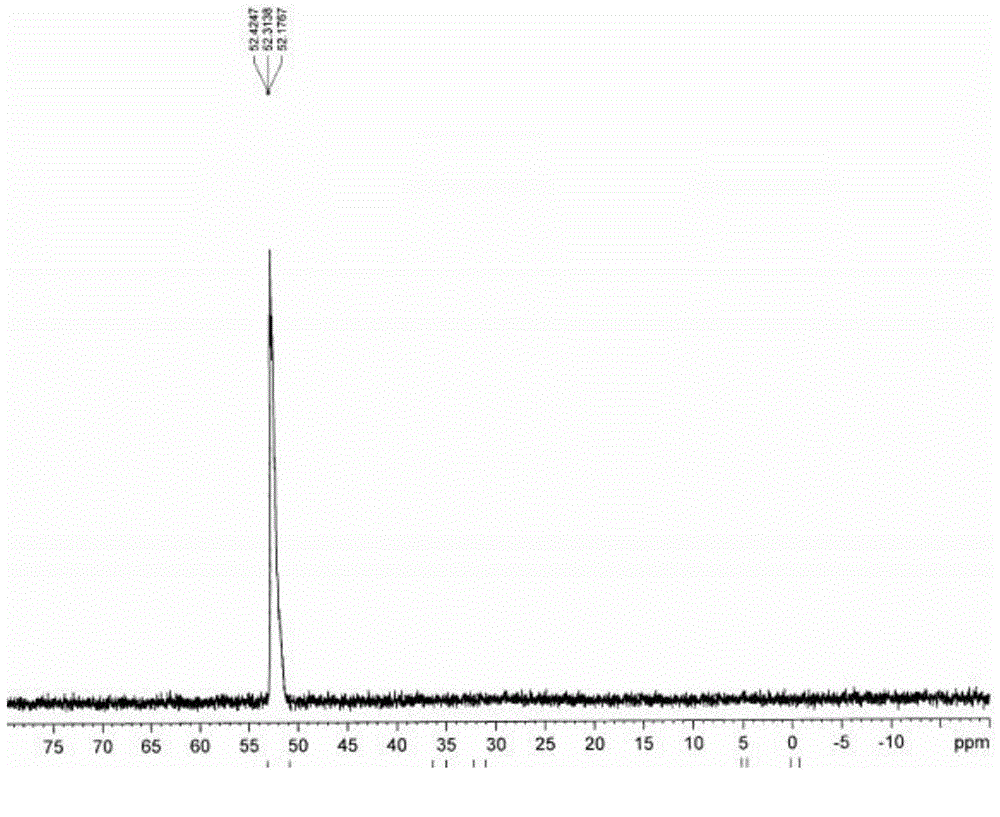
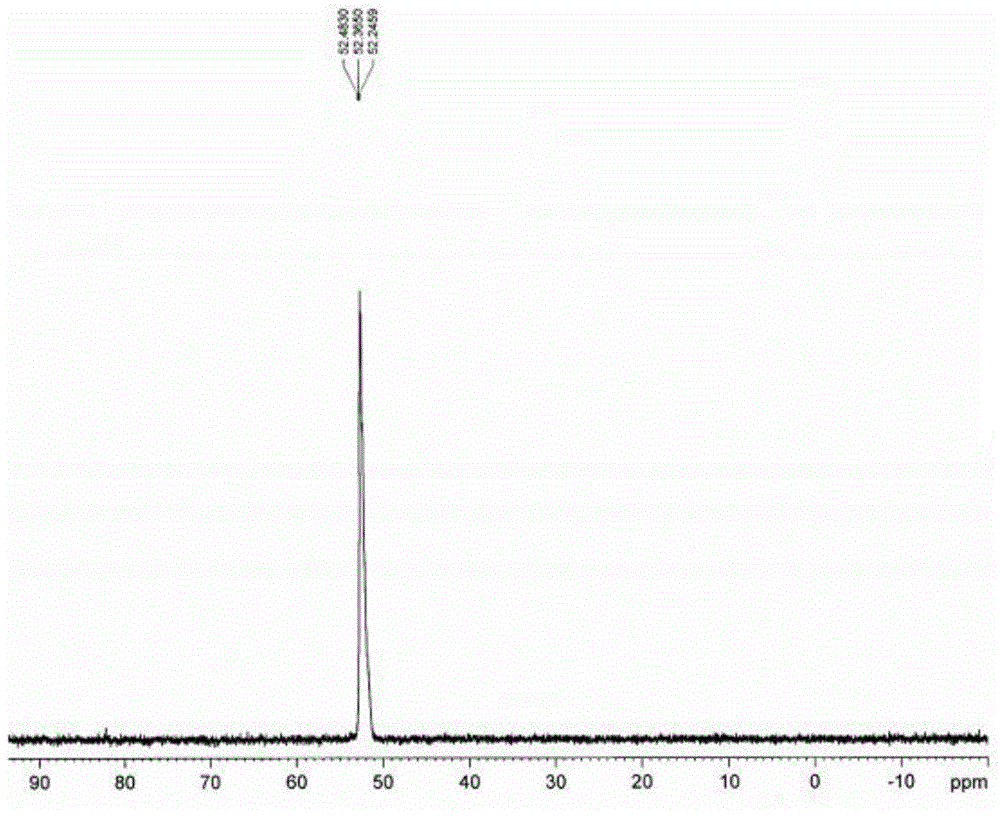
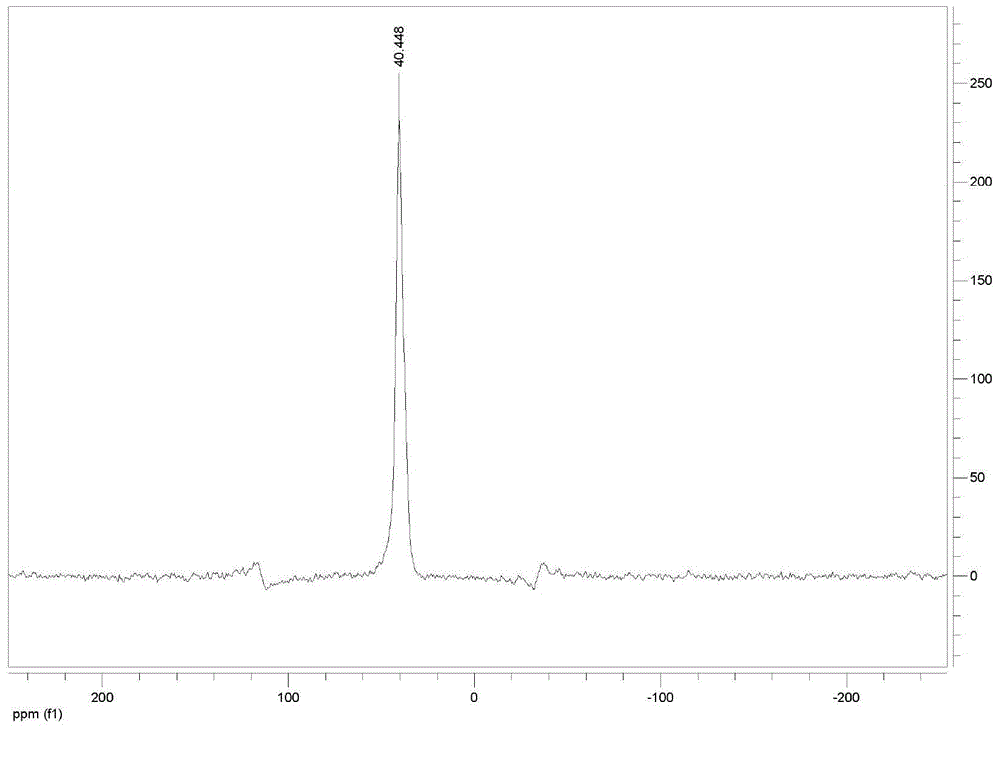
Preparation method of diethyl phosphinic acid without monoethyl phosphinic acid group and salt thereof
A technology of monoethylphosphinic acid and diethylphosphinic acid, which is applied in the field of preparation of diethylphosphinic acid and its salts, can solve the problems of complex process, high cost, waste water, etc., and achieve good removal effect, Ease of recycling and simple waste liquid treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Dissolve sodium hypophosphite monohydrate (2mol) and acetic anhydride (2mol) in 44ml of ethylene glycol monomethyl ether, then add a catalytic amount of glacial acetic acid (0.1mol) under stirring, stir at room temperature for 2 hours, then add anhydrous Sulfuric acid (1mol), after adding anhydrous sulfuric acid, cool down to 15°C and let it stand for 1 hour, filter and recover sodium sulfate, and obtain anhydrous hypophosphorous acid solution;
[0057] Transfer the anhydrous hypophosphorous acid solution into an acid-resistant and pressure-resistant reaction kettle with a constant pressure dropping device, feed ethylene gas, and make the pressure inside the kettle reach 1MPa, and distribute it from the constant pressure dropping device under stirring at a speed of 1800r / min. Add in batches the initiation mixture formed by mixing 20g of azobisisobutyronitrile, 0.4g of copper complex and 44ml of ethylene glycol monomethyl ether, and keep the ethylene pressure in the react...
Embodiment 2
[0065] Sodium hypophosphite monohydrate (2mol) and acetic anhydride (2mol) were dissolved in 44ml of ethylene glycol monomethyl ether, then a catalytic amount of propionic acid (0.05mol) was added under stirring, and 98% Concentrated sulfuric acid (1mol), after adding 98% concentrated sulfuric acid, cool down to 10°C and let it stand for 2.5 hours, filter and recover sodium sulfate, and obtain anhydrous hypophosphorous acid solution;
[0066] Transfer the anhydrous hypophosphorous acid solution into an acid-resistant and pressure-resistant reaction kettle with a constant pressure dropping device, feed ethylene gas, and make the pressure inside the kettle reach 1.2MPa, and stir from the constant pressure dropping device at a speed of 800r / min. Add in batches the initiation mixture formed by mixing 20g of azobisisobutyronitrile, 0.4g of copper complex and 44ml of ethylene glycol monomethyl ether, and keep the ethylene pressure in the reactor not lower than 0.6 during the dropwise...
Embodiment 3
[0073] Dissolve sodium hypophosphite monohydrate (2mol) and acetic anhydride (2.2mol) in 55ml of dioxane, then add a catalytic amount of isovaleric acid (2mol) under stirring, and then add 98.4% of Concentrated sulfuric acid (1.2mol), after adding concentrated sulfuric acid, cool down to 5°C and let stand for 6 hours, filter and recover sodium sulfate, and obtain anhydrous hypophosphorous acid solution;
[0074] Transfer the anhydrous hypophosphorous acid solution into an acid-resistant and pressure-resistant reaction kettle with a constant pressure dropping device, feed ethylene gas, and make the pressure inside the kettle reach 0.1MPa, and stir from the constant pressure dropping device at a speed of 1800r / min. Add in batches the initiation mixture formed by mixing 20g of azobisisobutyronitrile, 0.4g of copper complex and 44ml of ethylene glycol monomethyl ether, and keep the ethylene pressure in the reactor not lower than 0.1 during the dropwise addition of the initiation mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com