A spirobifluorenoindole derivative, its preparation method and application
A technology of spirobifluorene and its derivatives, which is applied in the fields of spirobifluorene and indole derivatives, their preparation and application, can solve the problems of poor stability and low efficiency of blue light materials, achieve reduced intermolecular aggregation, high yield, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
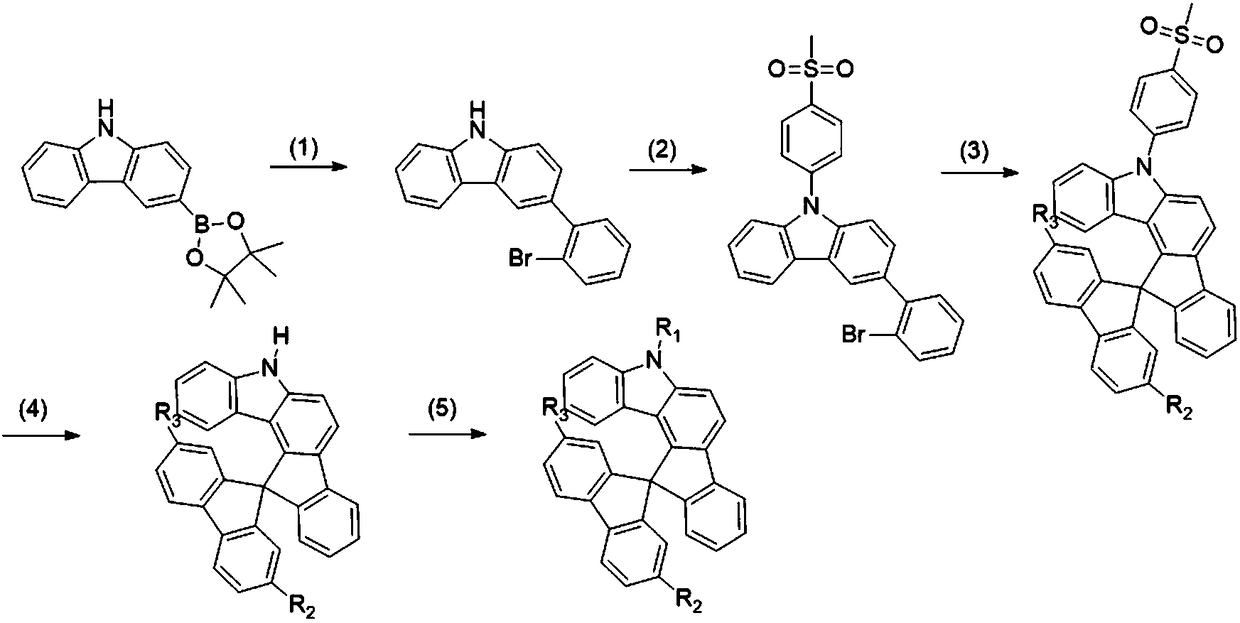
[0069] The preparation method of the spirobifluorenoindole derivatives of the present invention, the synthetic route schematic diagram is as follows figure 1 shown, including the following steps:
[0070] (1) Suzuki cross-coupling reaction: 3-carbazole boronic acid pinacol ester and o-dibromobenzene undergo Suzuki cross-coupling reaction to obtain the intermediate product 3-(2-bromophenyl)-9H-carbazole;
[0071] (2) Adding a protecting group: the intermediate product 3-(2-bromophenyl)-9H-carbazole obtained in step (1) is used as a reactant, and N,N-dimethylformamide (DMF) is used as a reaction solvent , use sodium hydride to extract hydrogen, then add p-toluenesulfonyl chloride, the molar ratio of 3-(2-bromophenyl)-9H-carbazole, p-toluenesulfonyl chloride and sodium hydride is 1:1 ~2:1~3, react at room temperature to obtain the intermediate product 3-(2-bromophenyl)-9-(4-(methylsulfonyl)phenyl)-9H-carbazole;
[0072] (3) Formation of spirobifluorenoindole: Dissolve the inter...
Embodiment 1
[0085] The spirobifluorene indole derivative 34 (5-(4,6-diphenyl-1,3,5-triazine)-5H spirofluorene-9,8-indenecarbazole) of the present invention can be Synthesized by the following method.
[0086]
[0087] (1) Suzuki cross-coupling reaction: In a dry 500ml two-necked flask, 3-carbazole boronic acid pinacol ester (15g, 51mmol), o-dibromobenzene (14.5g, 61.2mmol), toluene (120mL ), ethanol (60mL) and 2mol / L potassium carbonate solution (60mL) were added, ultrasonication was performed for 5-10 minutes first, and then nitrogen gas was stirred rapidly for 5 minutes, and the catalyst tetrakis(triphenylphosphine) palladium (1.8g, 1.53 mmol), a large amount of nitrogen was passed for 10 minutes. Heated to 100°C and stirred at reflux for 12h. During treatment, extract first, spin dry, and use petroleum ether and dichloromethane column chromatography to obtain a white solid product 3-(2-bromophenyl)-9H-carbazole with a yield of 93%.
[0088] (2) Adding a protecting group: first ad...
Embodiment 2
[0096] The spirobifluorene-indole derivatives prepared in Example 1 have the formula 34(5-(4,6-diphenyl-1,3,5-triazine)-5H spirofluorene-9,8-indencarba Azole) was used as the light-emitting guest to prepare the device.
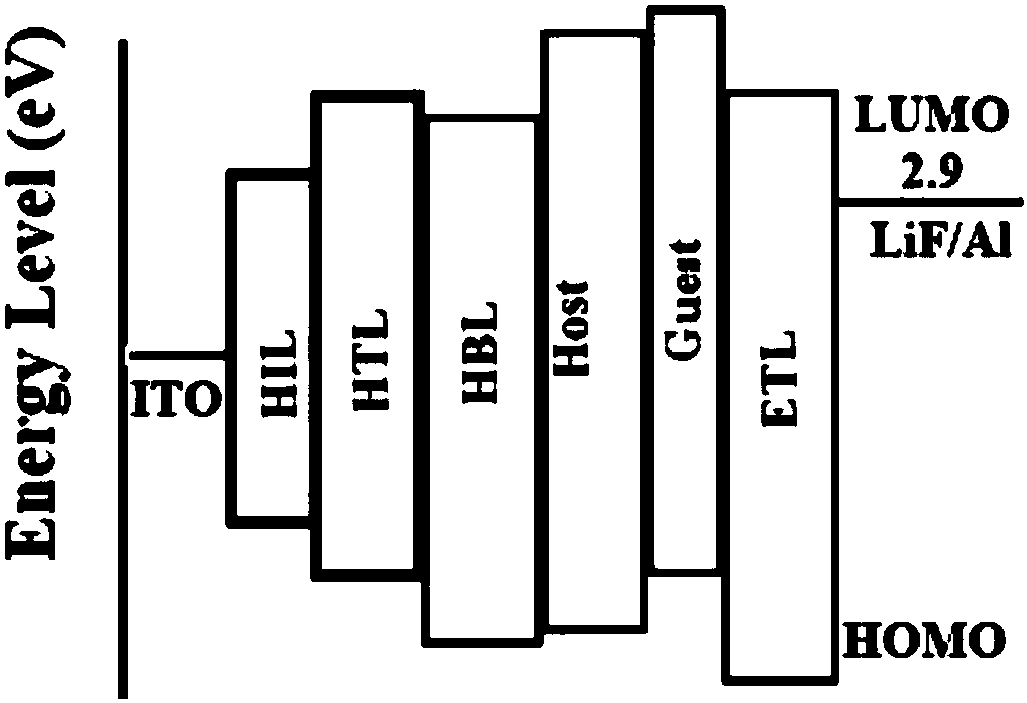
[0097] This example demonstrates the performance verification of an electroluminescent device fabricated with 34 as a guest luminescent material. The ITO (Indium Tin Oxide) glass was ultrasonically cleaned in detergent and deionized water for 30 minutes sequentially. Then vacuum dry for 2 hours (105°C), then put the ITO glass into the plasma reactor for 5 minutes of oxygen plasma treatment, transfer it to the vacuum chamber to prepare organic film and metal electrode, and then prepare a layer of 10nm by vacuum evaporation. The hole injection material molybdenum trioxide, followed by evaporation of 60nm thick hole transport material: 4,4'-cyclohexyl bis[N,N-bis(4-methylphenyl)aniline] (TAPC), and then evaporation Plating 4,4',4"-tris(carbazol-9-yl)triphenylam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com