Application of β-glucosidase in converting total flavonoids of Epimedium to prepare baochoside i
A technology of glucosidase and epimedium, applied in the directions of glycosylase, biochemical equipment and methods, enzymes, etc., can solve the problems of increased cost, single biotransformation method, and the efficiency of icariin needs to be improved, etc. High expression, excellent temperature stability and high transformation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Obtaining the β-glucosidase gene of the present invention and constructing the recombinant plasmid pET-DthBGL3
[0023] 1.1 Cultivation of Dictyoglomus thermophilum DSM 3960
[0024] Dictyoglomus thermophilum DSM 3960 was purchased from DSMZ Culture Collection (www.dsmz.de) and the number is 13995. The medium formula is: potassium dihydrogen phosphate 1.5g / L, disodium hydrogen phosphate dodecahydrate 4.2g / L, chlorine Ammonium chloride 0.5g / L, magnesium chloride hexahydrate 0.38g / L, calcium chloride dihydrate 0.06g / L, ferric ammonium sulfate hexahydrate 0.04g / L, cobalt chloride hexahydrate 2.9mg / L, sodium molybdate dihydrate 2.4mg / L, sodium selenate pentahydrate 1.7mg / L, manganese chloride tetrahydrate 2mg / L, zinc sulfate 2.8mg / L, soluble starch 5g / L, peptone 2g / L, yeast extract 2g / L, carbonic acid Sodium 1g / L, cysteine hydrochloride 1g / L, resazurin sodium 1g / L, deoxygenated in a nitrogen environment, adjusted the pH to 7.2. Inoculate with a syringe according t...
Embodiment 2
[0039] Example 2: Preparation of β-glucosidase Dth3 of the present invention
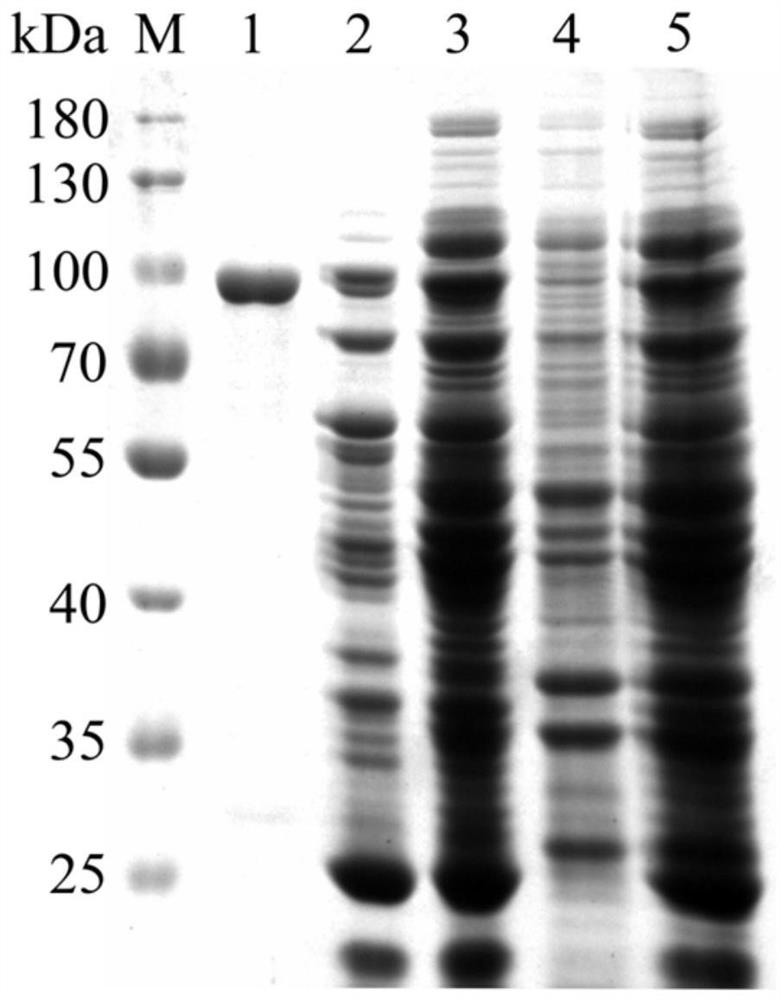
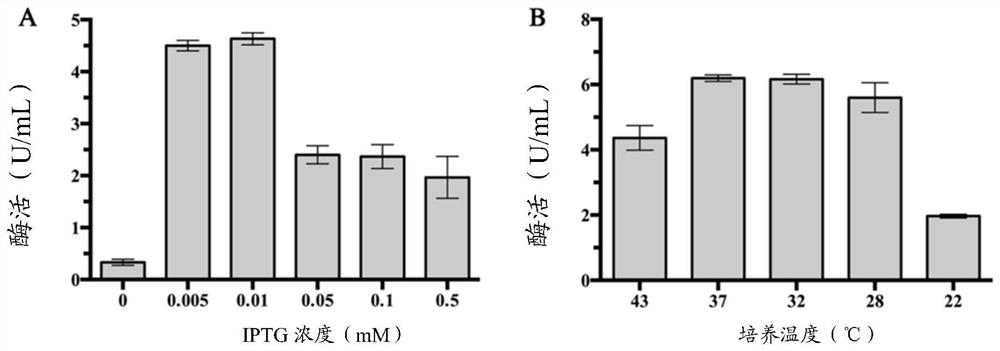
[0040] The recombinant plasmid pET-DthBGL3 was transformed into Escherichia coli JM109(DE3) host strain (purchased from Novagen), on an LB plate containing kanamycin (50μg / mL) (LB medium: tryptone 10g / L, yeast extract 5g / L, NaCl 5g / L, agar 15g / L) cultured overnight at 37°C, pick the transformants into 200mL LB medium (50μg / mL kanamycin) 37°C, 200rpm shaking culture until OD600 is 0.6 Add the final concentration of 0.005-0.01mM isopropyl β-D-thiogalactopyranoside (IPTG) inducer, incubate at 30°C for 6h, and use a high-speed refrigerated centrifuge to put the culture solution at 4°C to 13,000 Centrifuge at rpm for 15 min to collect the bacteria.
[0041] Since the recombinant plasmid pET-DthBGL3 contains a His-tag tag, it is purified by His·Bind Purification Kit (purchased from Novagen) to obtain a purified recombinase. Specific operation process:
[0042] A. Sample handling
[0043] (1) Resuspend the wash...
Embodiment 3
[0058] Example 3: Qualitative determination of β-glucosidase of the present invention
[0059] 1. Determination method of enzyme activity
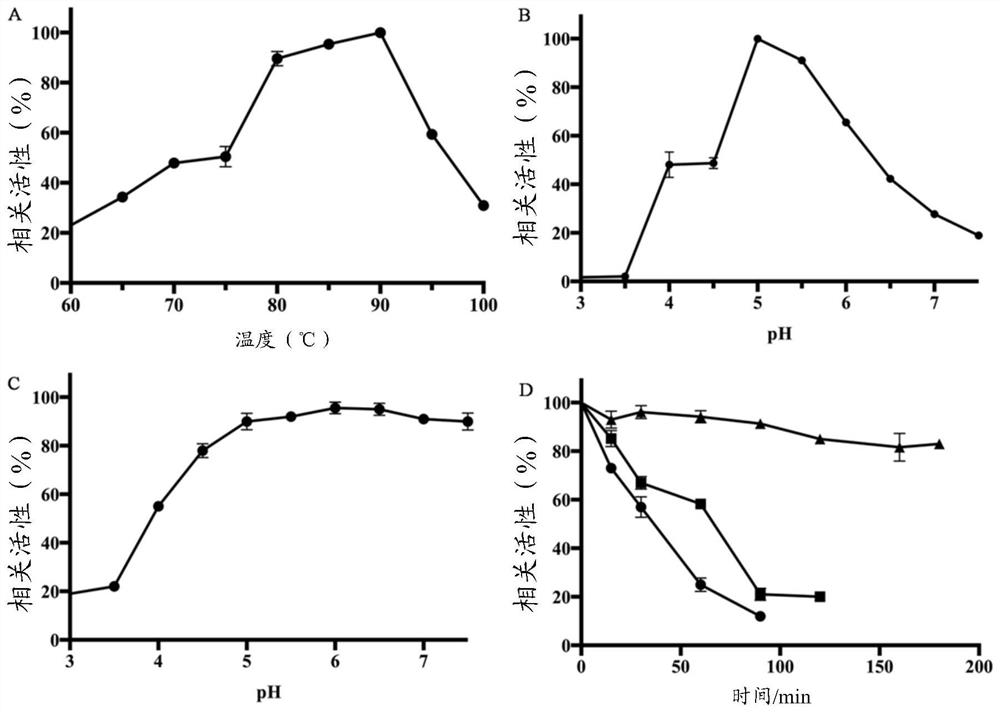
[0060] In the reaction system 100μL, 5μL 20mmol / L p-nitrophenyl-β-L-glucoside (pNPG), add 85μL 100mmol / L citric acid-disodium hydrogen phosphate buffer (pH 5.0), incubate at 90℃ for 2min, then add 10μL of the enzyme solution diluted to a suitable multiple was reacted for 10min, and then 600μL of 1mol / L sodium carbonate solution was added to stop the reaction after color development. The absorbance was measured at 405nm. Enzyme activity unit (U) is defined as: under the measurement conditions, the amount of enzyme required to produce 1 μmol p-nitrophenol per minute is 1 enzyme activity unit.
[0061] 2. Determination of the optimum reaction temperature
[0062] In the range of 60-100°C, the enzyme activity is measured every 5°C. The buffer is 100mmol / L citric acid-disodium hydrogen phosphate buffer, pH 5.0, the result is as follows figure 2 -A ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com