Preparation method and application of cortex albiziae lignan glycoside monomers
A kind of technology of lignan glycosides and Albizia Julibrissin bark, which is applied in the field of preparation of lignan glucoside monomers of Albizia Julibrissin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Isolation and Purification of Lignan Glycoside Monomer (Acanthoside E1) from the Bark of Albizia Julibrissin
[0031] 2.5kg of Albizia Julibrissin medicinal material was crushed into powder, refluxed and extracted twice in 75% ethanol at 75°C, the ratio of solid to liquid was 1:10 (g / mL), and the refluxed extraction time was 2h. The two extracts were combined, concentrated under reduced pressure, and dried in vacuo to obtain 250 g of the total extract of Albizia Julibrissin (AJ-T), and the yield of the crude product was 10%. The total extract was dissolved in distilled water, and then fractionally extracted with ethyl acetate and water-saturated n-butanol, respectively. The extract was concentrated under reduced pressure to obtain an ethyl acetate phase (124.7 g), n-butanol phase AJ-B (52.4 g), and a mother liquor aqueous phase (23.5 g).
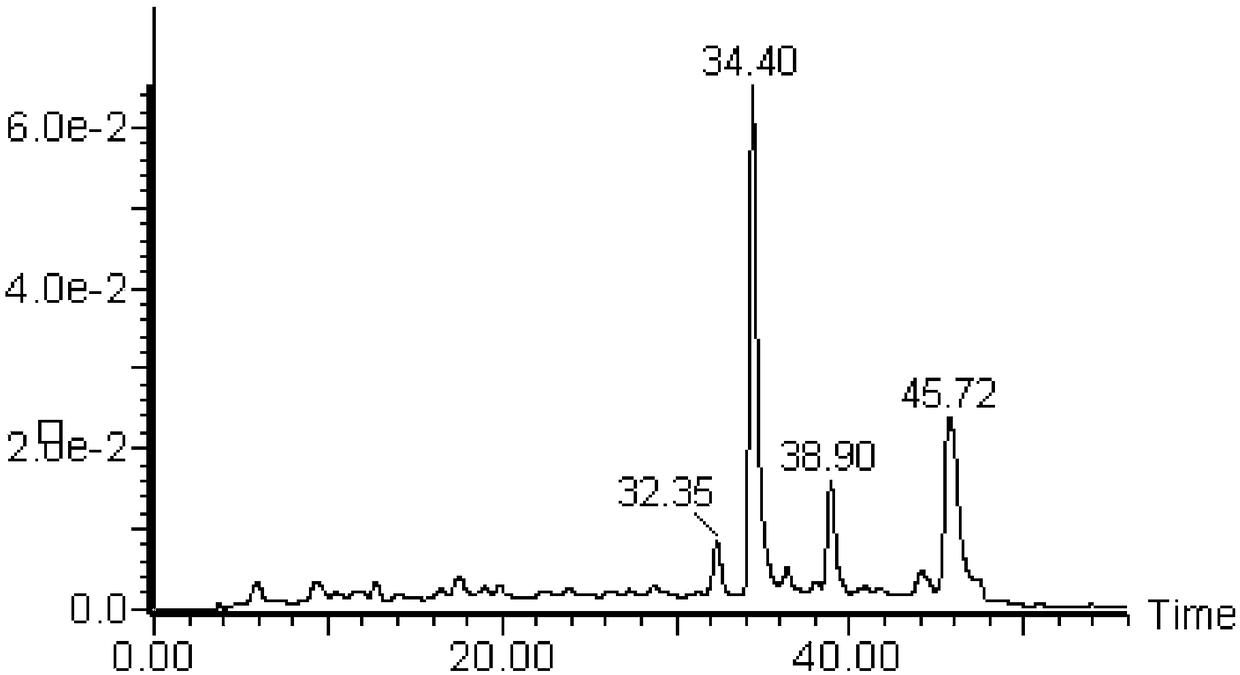
[0032] Take 20g of albizia juniper n-butanol dry powder, dissolve it in deionized water, elute the aqueous solution through a D101 ...
Embodiment 2
[0036] Structural identification of lignan glycoside monomer (eleutheroside E1) from the bark of Albizia Julibrissin
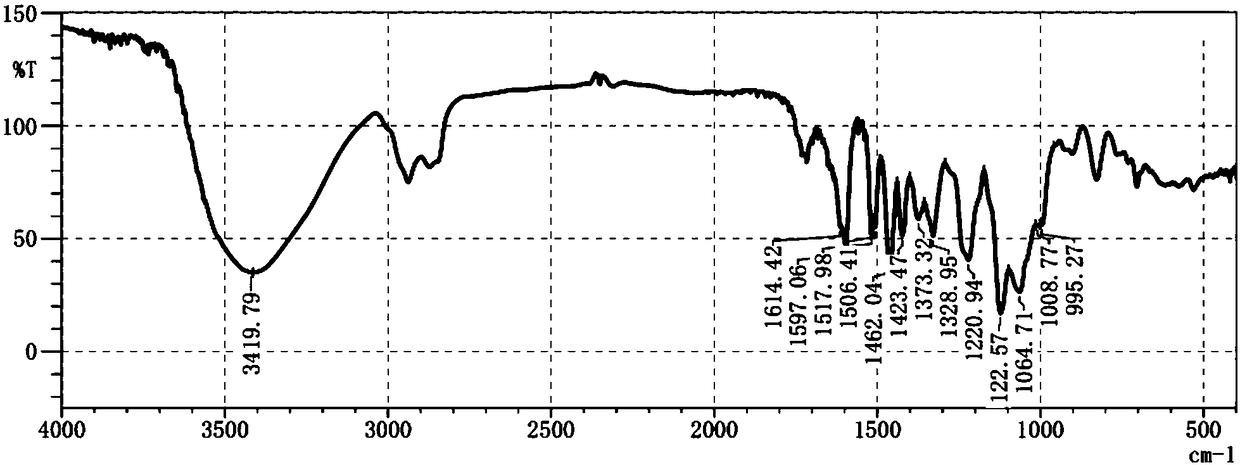
[0037] Sample H2 was dried, ground, and directly pressed into tablets with iodine bromide, detected by Fourier transform infrared spectrometer, and analyzed in the mid-infrared region (wavenumber 4000-400cm -1 ) change, record the infrared spectrum ( image 3 , the infrared spectrum of the sample), the IR spectrum results suggest that the compound contains a benzene ring, a carbon-oxygen bond or a hydroxyl group.
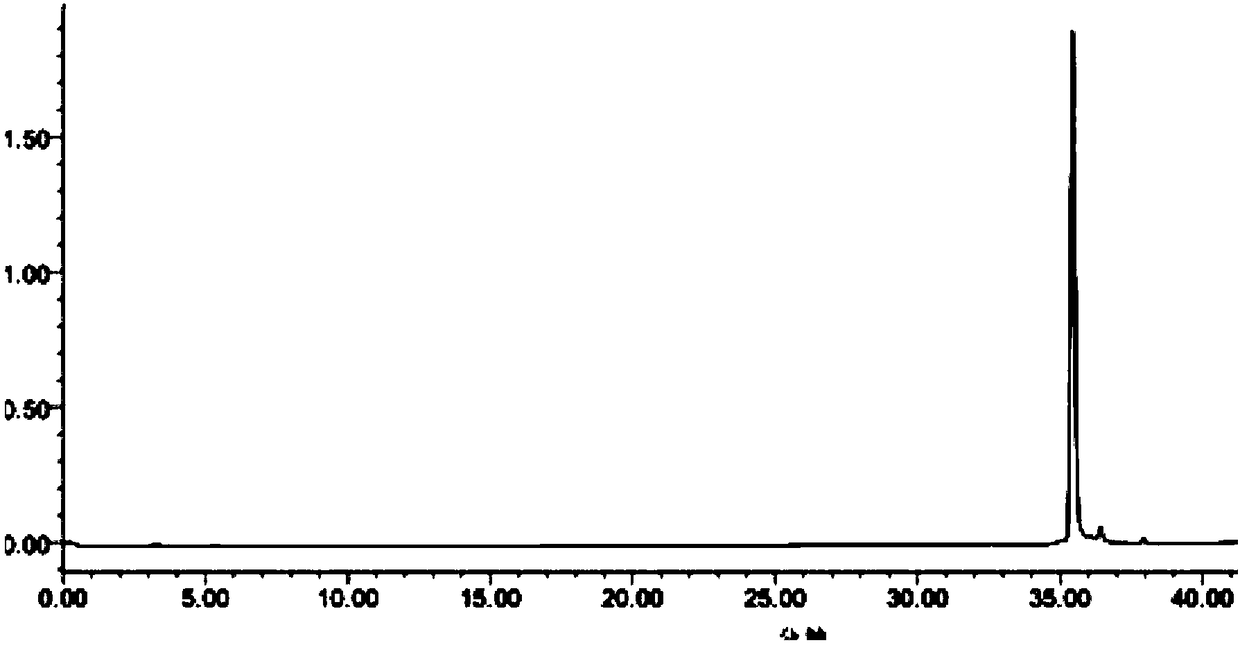
[0038] Mass Spectrometry Conditions: Ion Mode: ESI + ; Capillary voltage: 3.5kVolts; Cone voltage: 20Volts; Ion source temperature: 100℃; Desolvation temperature: 400℃; Mass range 100-1000m / z; Voltage: 1800Volts. MassLynx software was used to collect and process data, and the relative molecular mass of the compound was known to be 580. Multiple fragment ion peaks are generated simultaneously. ( Figure 4 , the mass spectrum of the sample)
[...
Embodiment 3
[0043] Detection of Fatty Acid Synthase Activity by UV-Vis Spectrophotometry
[0044] Fatty acid synthase supernatant was extracted and purified from chicken liver. NADPH has absorption at 340nm, but its product NADP has no absorption at 340nm. Therefore, the change of NADPH was determined by a microplate reader to determine the change of fatty acid synthase activity. Carried out in potassium phosphate buffer containing 1mmol / L EDTA, pH 7, concentration 0.1mmol / L, substrate concentration acetyl CoA 6μmol / L, malonyl CoA 12μmol / L, NADPH 37.5μmol / L, to be The concentration of the test sample is 50ug / mL, the total reaction volume is 2mL, after 4-5min in a 37°C water bath, add 50μL of diluted high-speed centrifuged fatty acid synthase supernatant to start the enzyme reaction, and absorb 200uL of the reaction system from the reaction system each time The solution was continuously monitored for changes in absorbance at a wavelength of 340 nm.
[0045] The activity unit (U) of anima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com