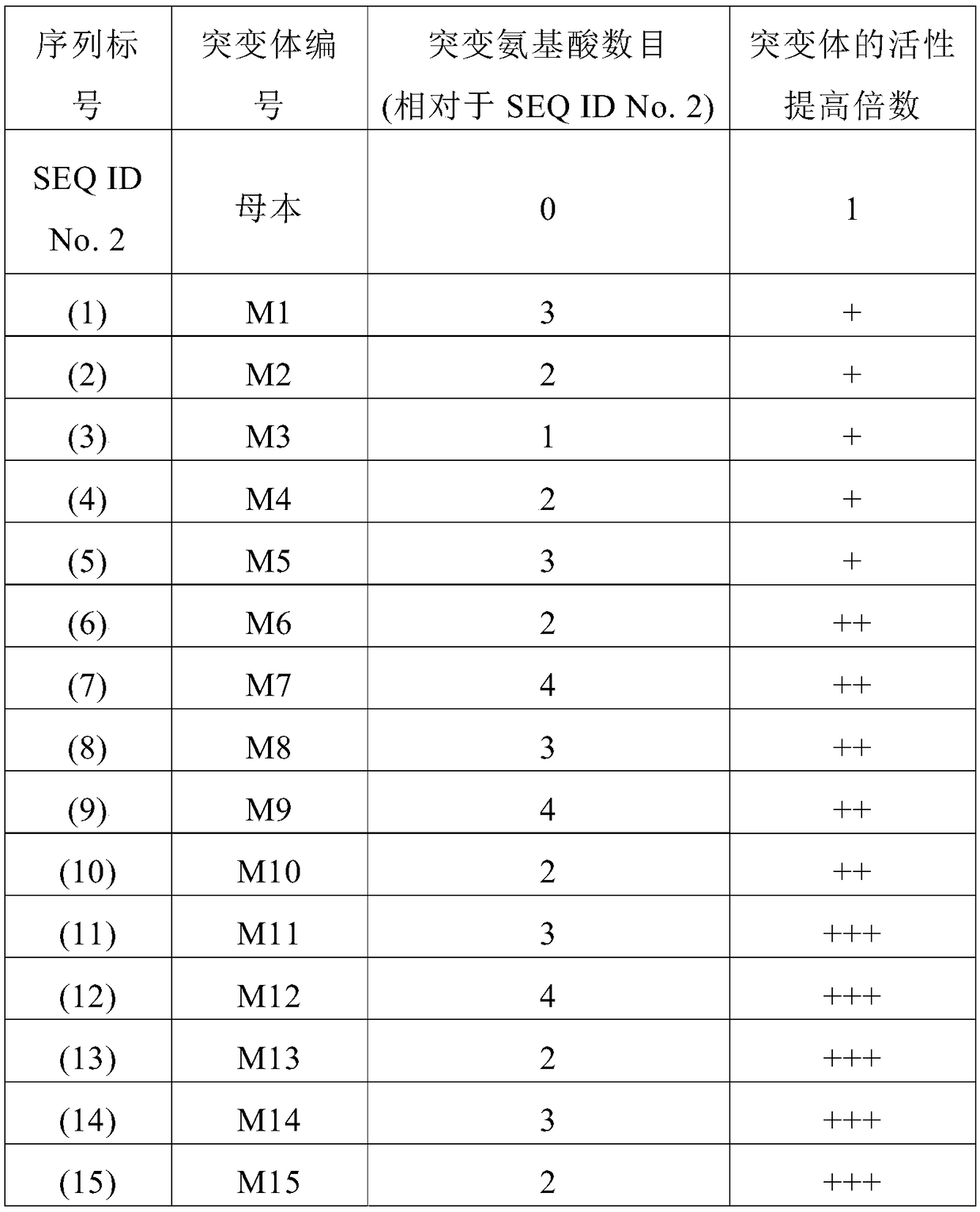
Carnosine hydrolase, gene, mutant and application thereof
A technology of hydrolase and carnosine, applied in the field of bioengineering, can solve the problems of low catalytic activity, no published L-carnosine yield data, and high cost of synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
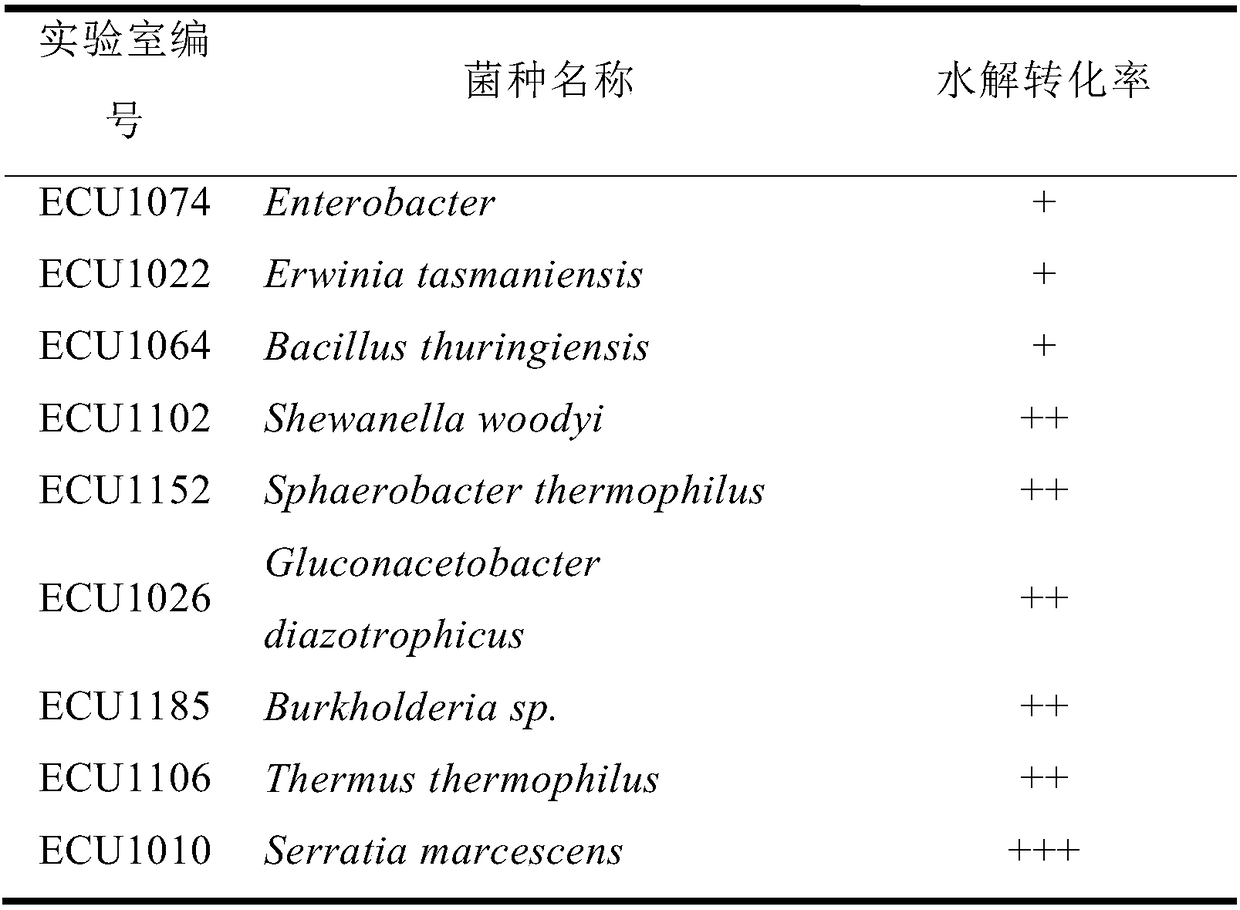
[0062] Example 1. Screening of carnosine hydrolase-producing bacteria
[0063] More than 500 strains preserved in the laboratory were cultured separately, the culture medium was centrifuged, cells were collected, and carnosine hydrolysis activity was screened. The preserved strains were inoculated into a test tube containing 4 mL of culture medium, and shaken at 30°C and 180rpm for 48h. After the culture was over, the cells were collected by centrifugation at 4°C and 8000rpm, and resuspended in Tris-HCl (50mM , pH8.0) buffer solution, at 30 ° C, 1000rpm shaking reaction for 24h, using high performance liquid chromatography to analyze the hydrolysis conversion rate of carnosine. The chromatographic column used is a chiral crown ether column (CR(+), φ40mm×150mm). The specific analysis conditions are: use perchloric acid solution with pH 1.0 as the mobile phase, the mobile phase flow rate is 0.3mL / min, and the detection wavelength is 220nm. temperature 25°C. The results are sho...
Embodiment 2
[0068] Embodiment 2. Cloning of carnosine hydrolase SmPepD gene
[0069] The strain Serratia marcescens ECU1010 was inoculated into LB medium for cultivation, and the total genomic DNA with high purity and large fragments was extracted by cetyltrimethylammonium bromide (CTAB) method. Add appropriate amount of Serratia marcescens cell into liquid nitrogen to freeze, grind into powder, add appropriate amount of 2×CTAB extraction buffer (100mmol / L Tris-HCl, pH 8.0, containing 20mmol / L EDTA, 1.4moI / L NaCl, 20g / L CTAB, 40mmol / L mercaptoethanol), at 65°C for 10 minutes, shaking intermittently. Then add an equal volume of chloroform / isoamyl alcohol, gently invert the centrifuge tube to mix, centrifuge at 12,000 rpm for 10 min at room temperature, transfer the supernatant to another centrifuge tube, add an equal volume of chloroform / isoamyl alcohol, and centrifuge upside down The tube was mixed well and centrifuged at 12000 rpm for 10 minutes at room temperature. Transfer the upper ...
Embodiment 3
[0074] Embodiment 3. Preparation of recombinant carnosine hydrolase SmPepD
[0075] Forward primer 5'-CCG GAATTC GTGTCTGAATTGTCTCAGCTTT-3', reverse primer 5'-CCG CTCGAG TTACGCGCGCTCAGGGATCGCTTT-3', the nucleotide sequence of the carnosine hydrolase SmPepD obtained in Example 2 is amplified by polymerase chain reaction technology, and the obtained amplified DNA fragment containing the SmPepD sequence is used with the restriction endonuclease EcoRI and XhoI double digestion, and then ligated with the plasmid pET28a that was also cut with restriction endonucleases EcoRI and XhoI to obtain the recombinant plasmid pET28a-SmPepD.
[0076] The obtained recombinant plasmid pET28a-SmPepD was transformed into Escherichia coli E.coli BL21, and the recombinant Escherichia coli was inoculated to the LB medium containing 50 μg / ml kanamycin (peptone 10g / L, yeast extract 5g / L, (NaCl 10g / L, pH 7.0), shake culture overnight at 37°C, transfer 1% (v / v) inoculum into a 2L Erlenmeyer flask conta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com