Treatment of immune disease by mucosal delivery of antigents using genetically modified lactobacillus
a technology of lactobacillus and immune disease, which is applied in the field of immune disease treatment by mucosal delivery of antigents using genetically modified lactobacillus, can solve the problems of no adequate treatment, no side effects, and huge cost, and achieves the activation of antigen-specific regulatory t cells, increased oral tolerance, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a
Induction of OVA-Specific Tolerance by Genetically Modified Lactococcus lactis Delivering OVA to OVA-Sensitized Wild Type Mice
Introduction
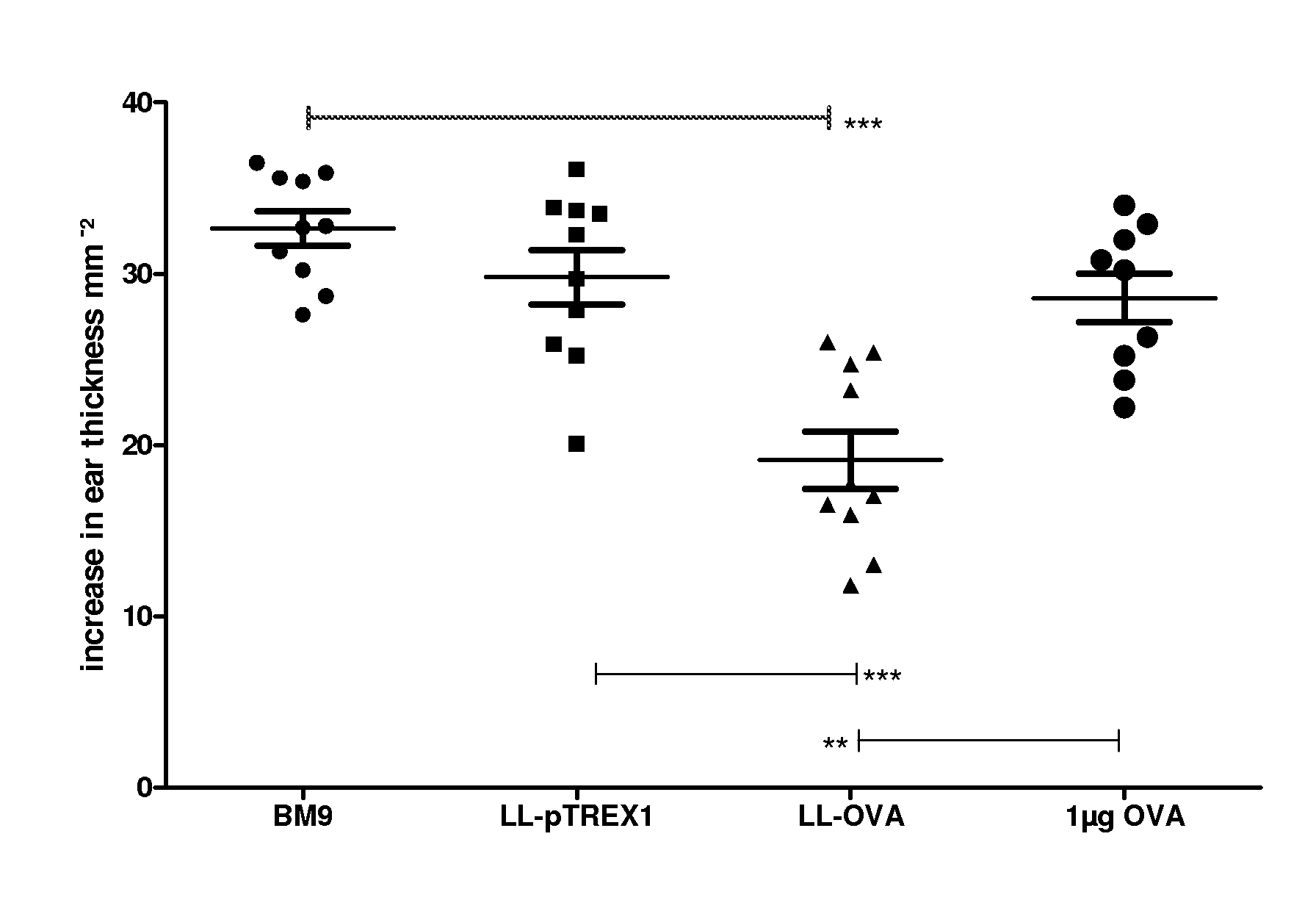
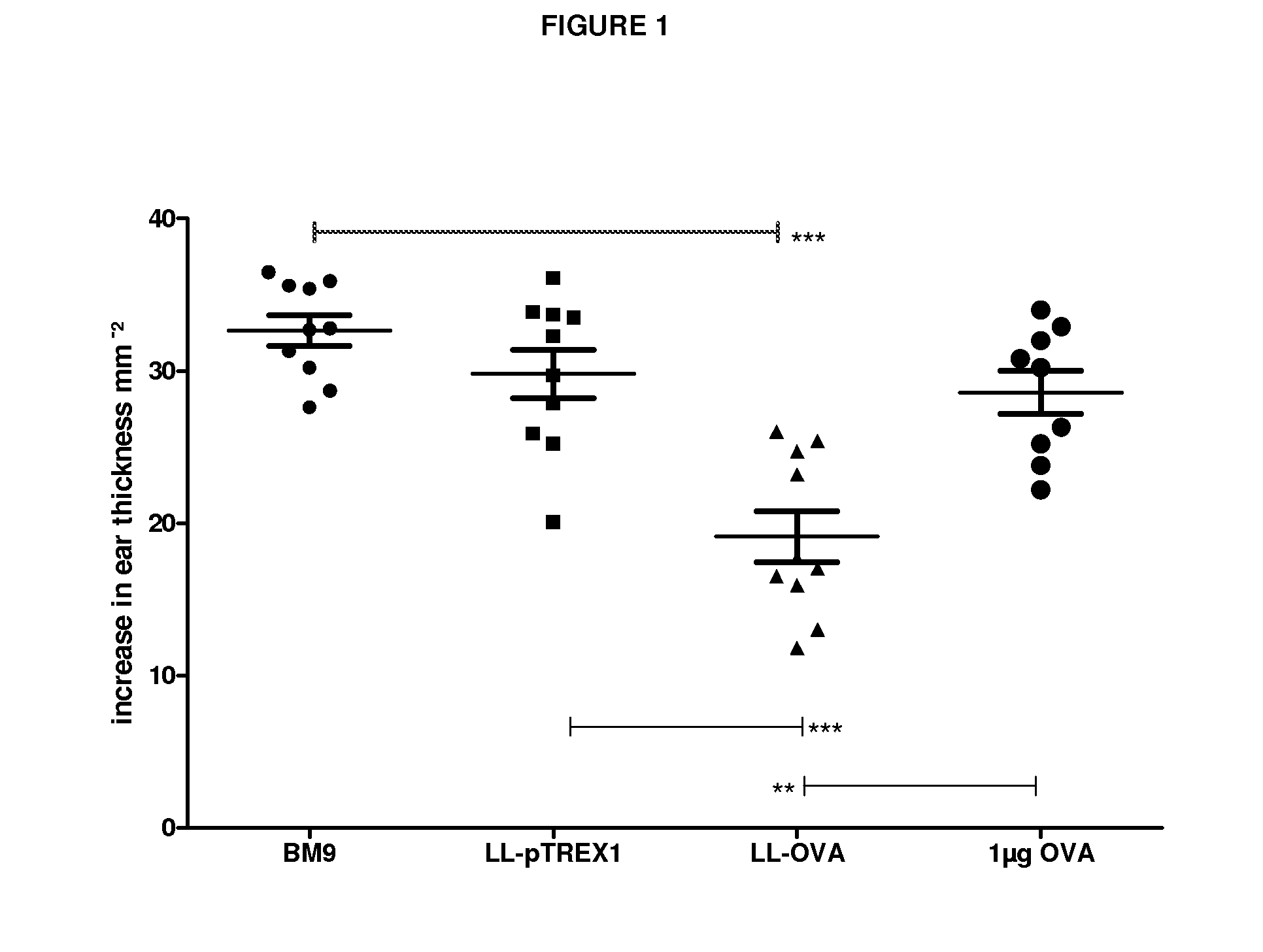
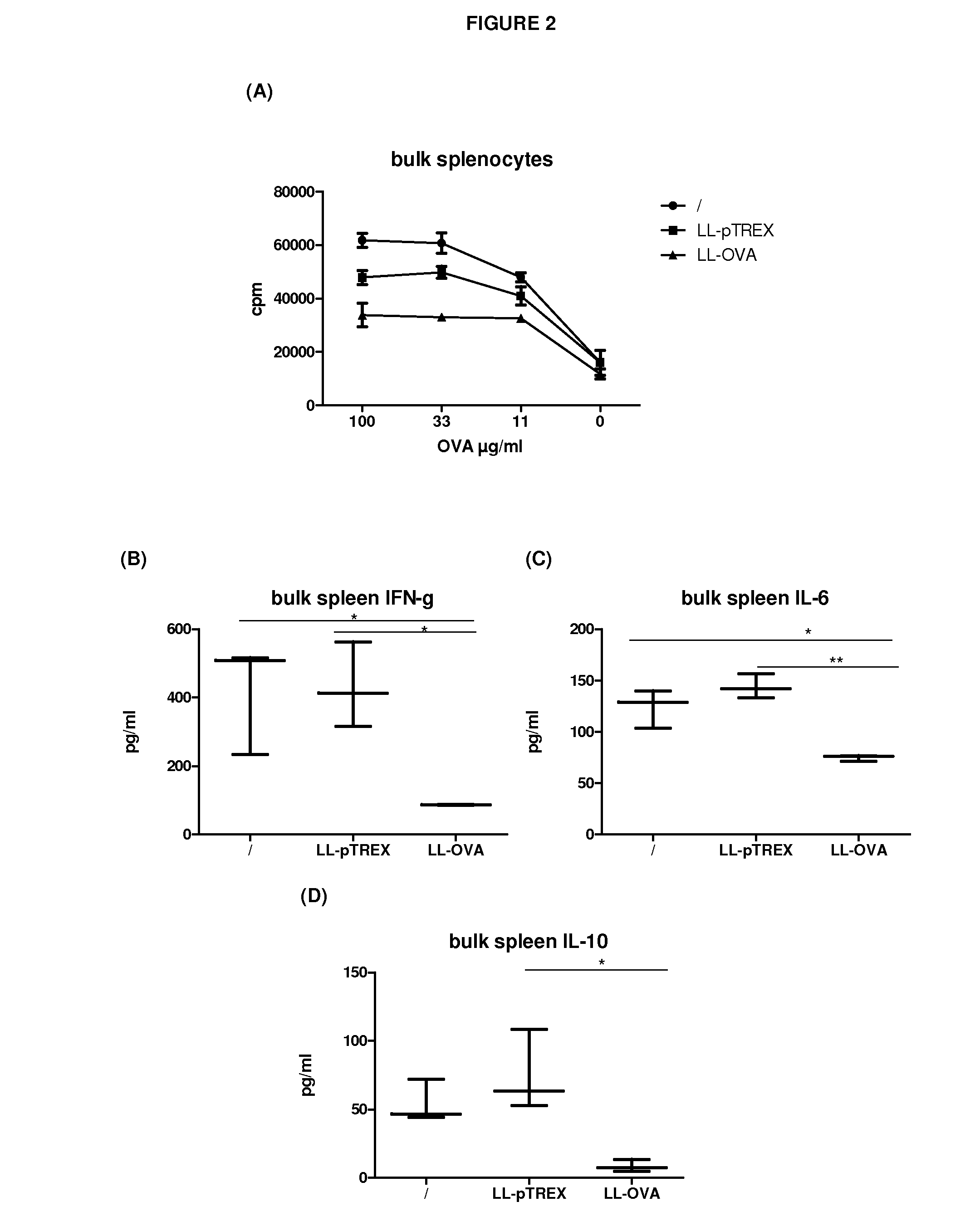
[0099]For this purpose we genetically engineered OVA secreting LL (LL-OVA) and evaluated the induction of systemic tolerance in a therapeutic model for autoimmunity / allergy, namely the OVA immunization model.
Materials and Methods
[0100]Bacteria and media: The Lactococcus lactis MG1363 (LL) strain was genetically modified and used throughout this study. Bacteria were cultured in GM17E medium consisting of M17broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% glucose and 5 μg / ml erythromycin (Abbott). Stock suspensions of LL strains were stored at −20° C. in 50% glycerol in GM17E medium. Stock suspensions were diluted 500-fold in GM17E medium and incubated at 30° C. overnight. Within 16 h they reached a saturation density of 2×109 colony forming units (CFU) per ml. Bacteria were harvested by centrifugation and resuspended in BM9 medium...
example b
Induction of Antigen-Specific Oral Tolerance by Genetically Modified Lactococcus lactis Delivering DQ8-Specific Immunodominant Gliadin Epitopes to Gluten-Sensitized Class II Transgenic Mice
Introduction
[0115]Celiac disease, also known as celiac sprue or gluten-sensitive enteropathy, is a chronic inflammatory disease that develops from an immune response to specific dietary grains that contain gluten. Celiac is a complex multigenic disorder that is strongly associated with the genes that encode the human leukocyte antigen variants HLA-DQ2 or HLA-DQ8. One of the most important aspects in the pathogenesis of Celiac is the activation of a T-helper 1 immune response. This arises when antigen-presenting cells that express HLA-DQ2 / DQ8 molecules present the toxic gluten peptides to CD4(+) T-cells. Both classes of gluten proteins, gliadins and glutenins, contain peptides that bind DQ2 and DQ8. It is generally accepted that the immune response, such as the production of IFN-γ from gluten-speci...
example c
Induction of Tolerance to Clotting Factor VIII and Factor IX Following Oral Administration of L. lactis Secreting Said Factors
Introduction
[0138]Several therapeutic (recombinant) proteins, such as interferon's, factor VIII / IX and antibodies (Remicade) are administered at high doses over prolonged treatment periods. However, a complication associated with their use is the development of protein-specific immune responses, such as antibodies. These antibodies (Abs), also called inhibitors, render the therapeutic proteins less effective. Examples include the formation of inhibitors for factor VIII / IX in hemophilia, erythropoietin (Epo) in patients undergoing therapy for chronic renal failure, and IFN- in patients undergoing treatment for multiple sclerosis. Here, we demonstrate that oral delivery of the Factor VIII (and Factor IX) by L. lactis suppresses inhibitor formation to said factor via the induction of antigen-specific CD4+ regulatory T cells.
Material and Methods
[0139]Bacteria and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com