Patents
Literature
80 results about "Internet portal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
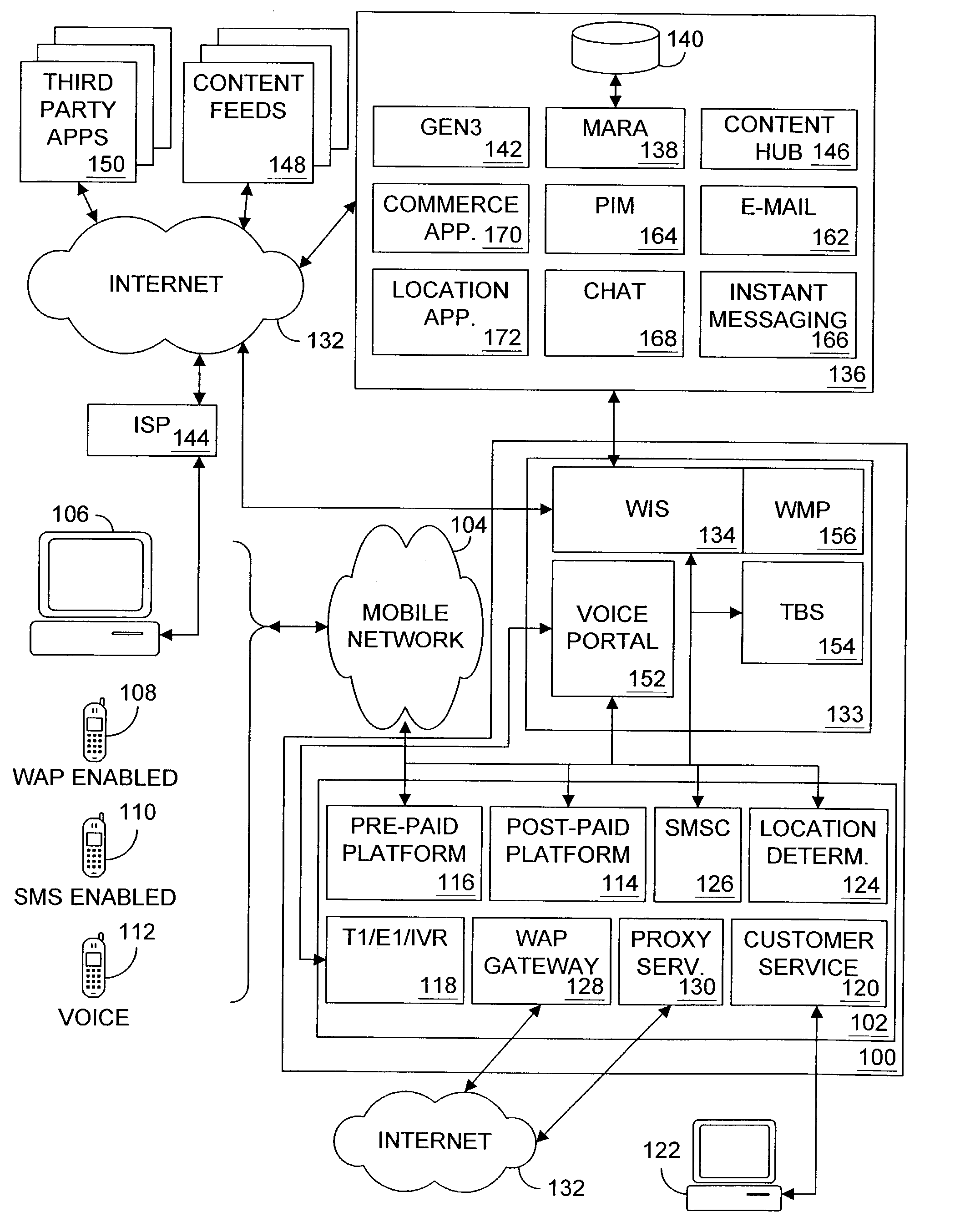
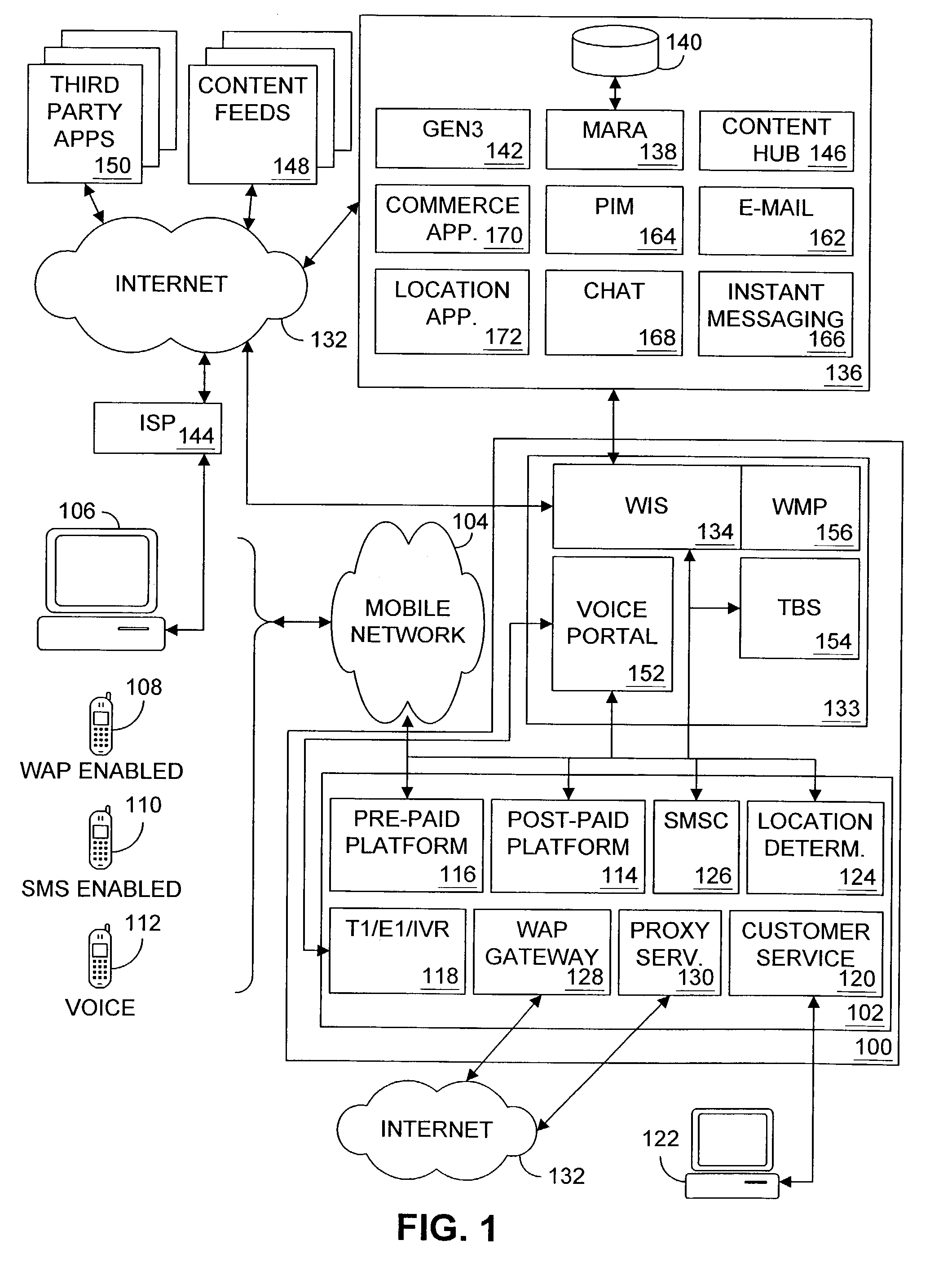
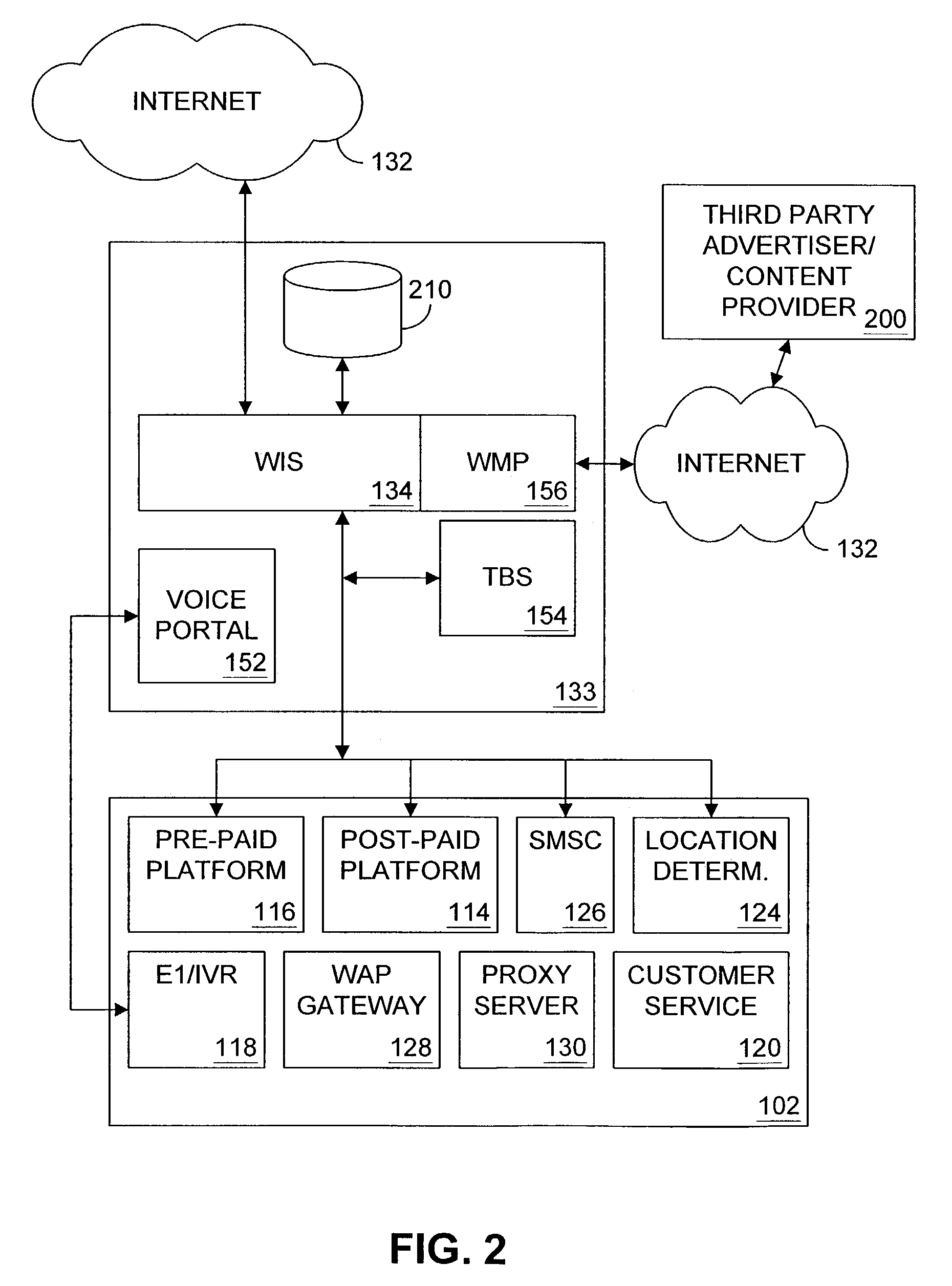
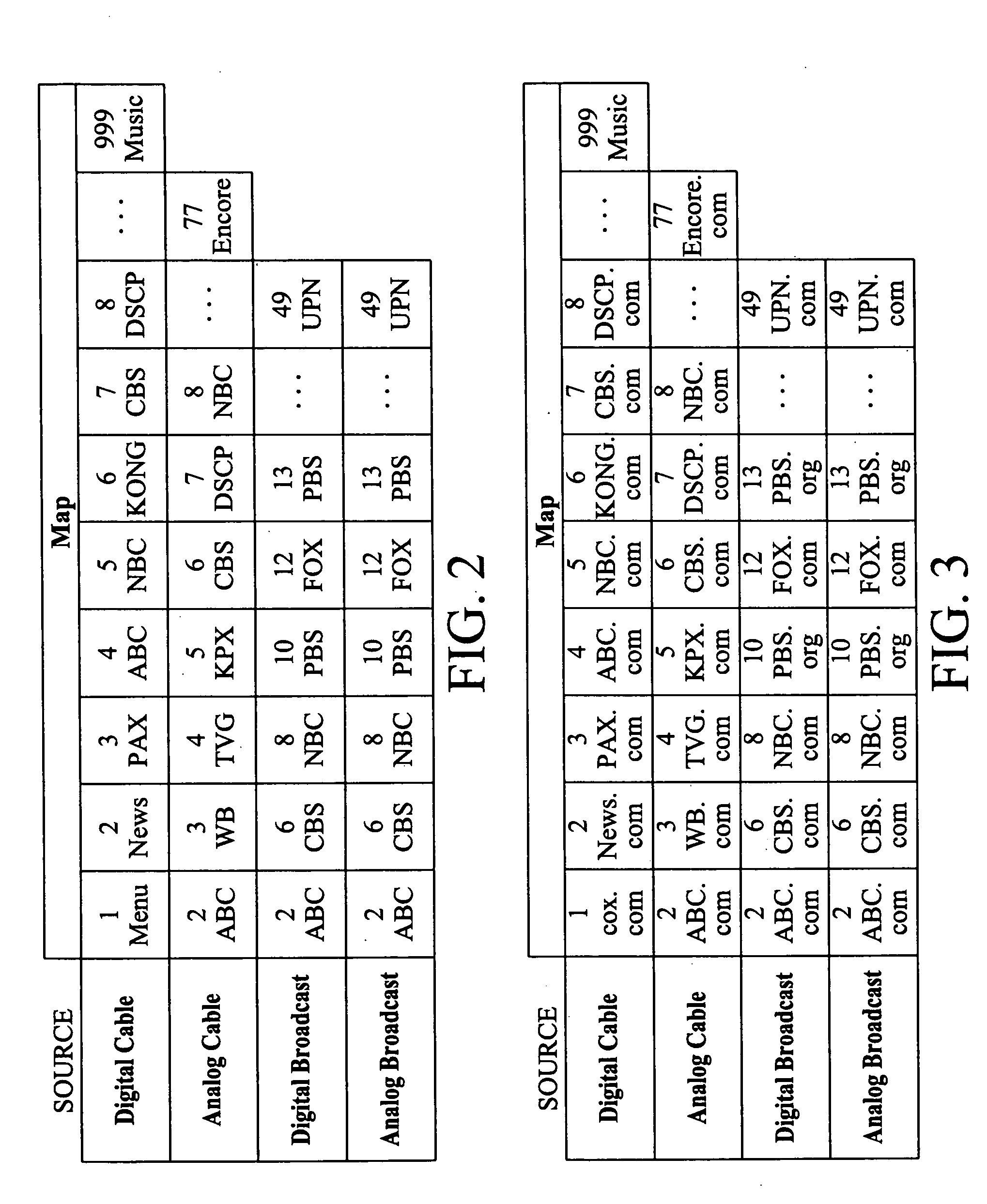
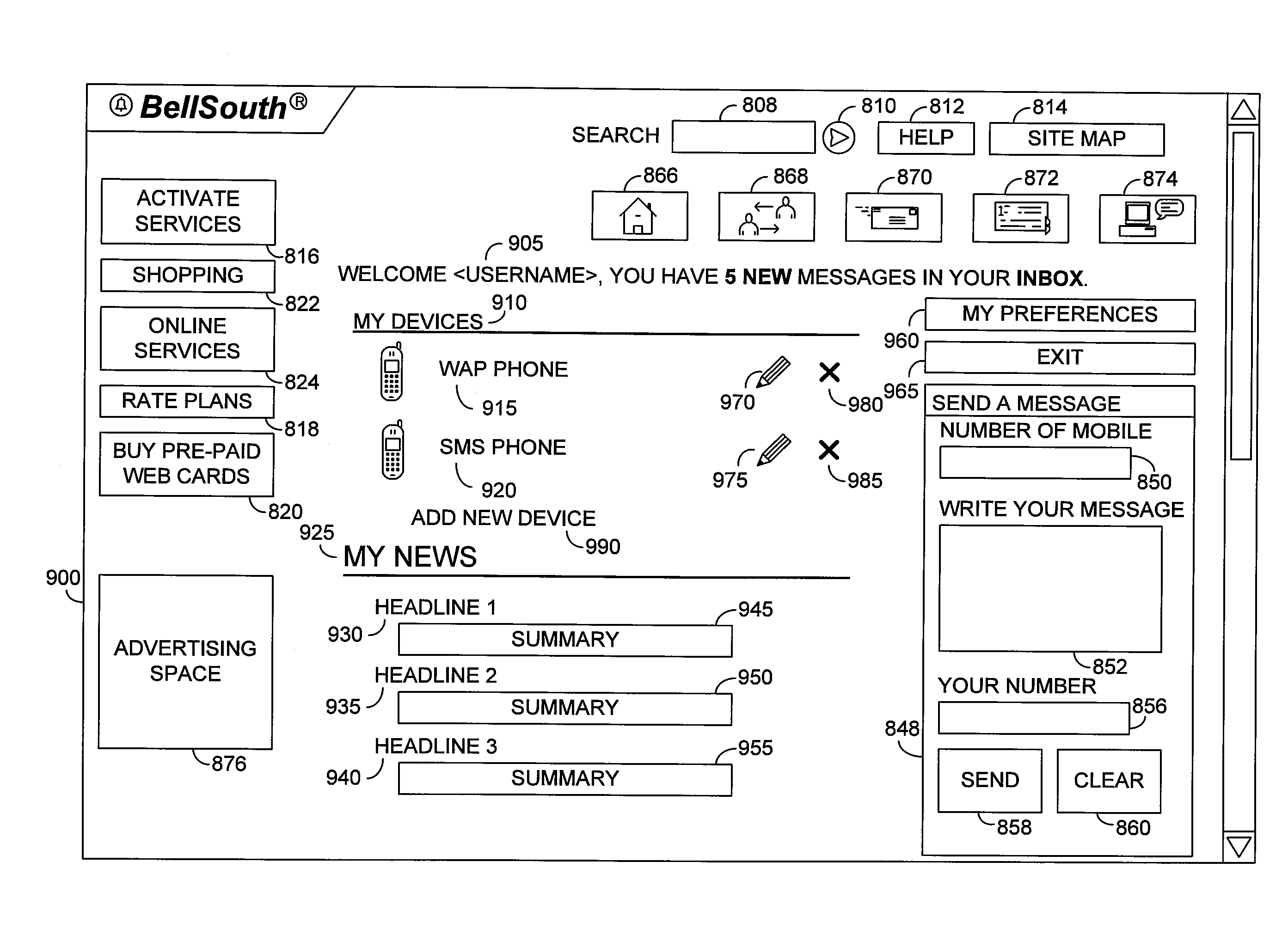
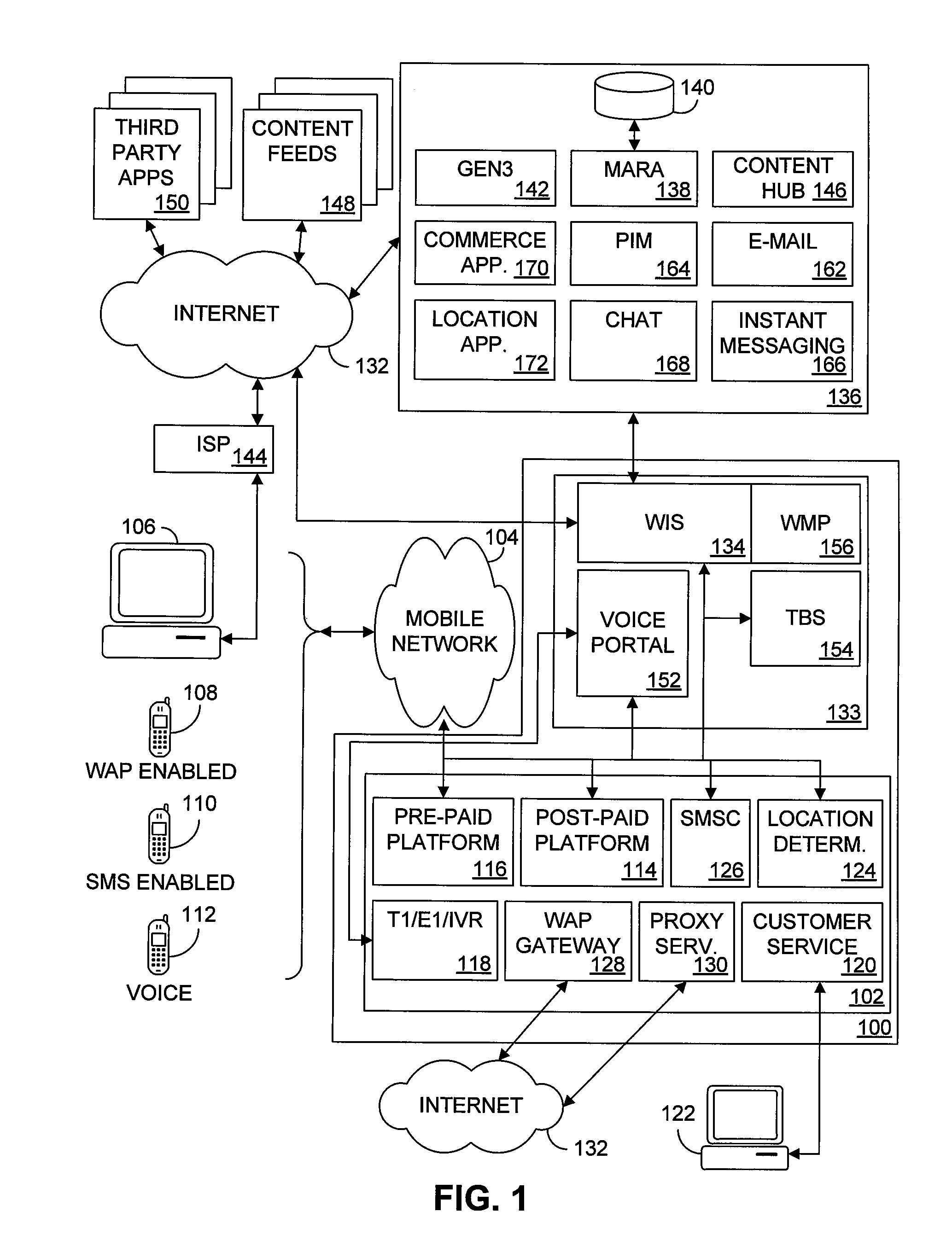
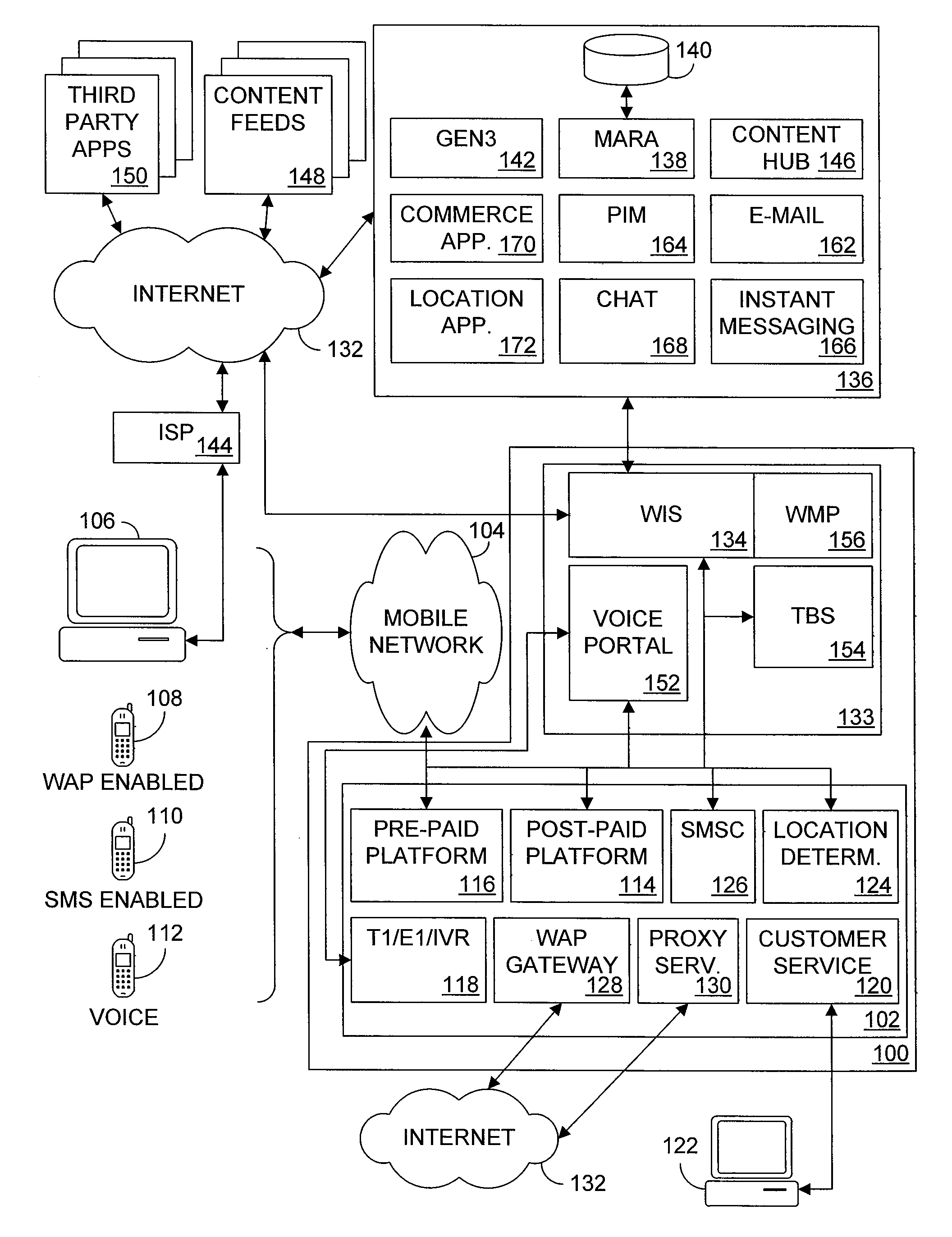
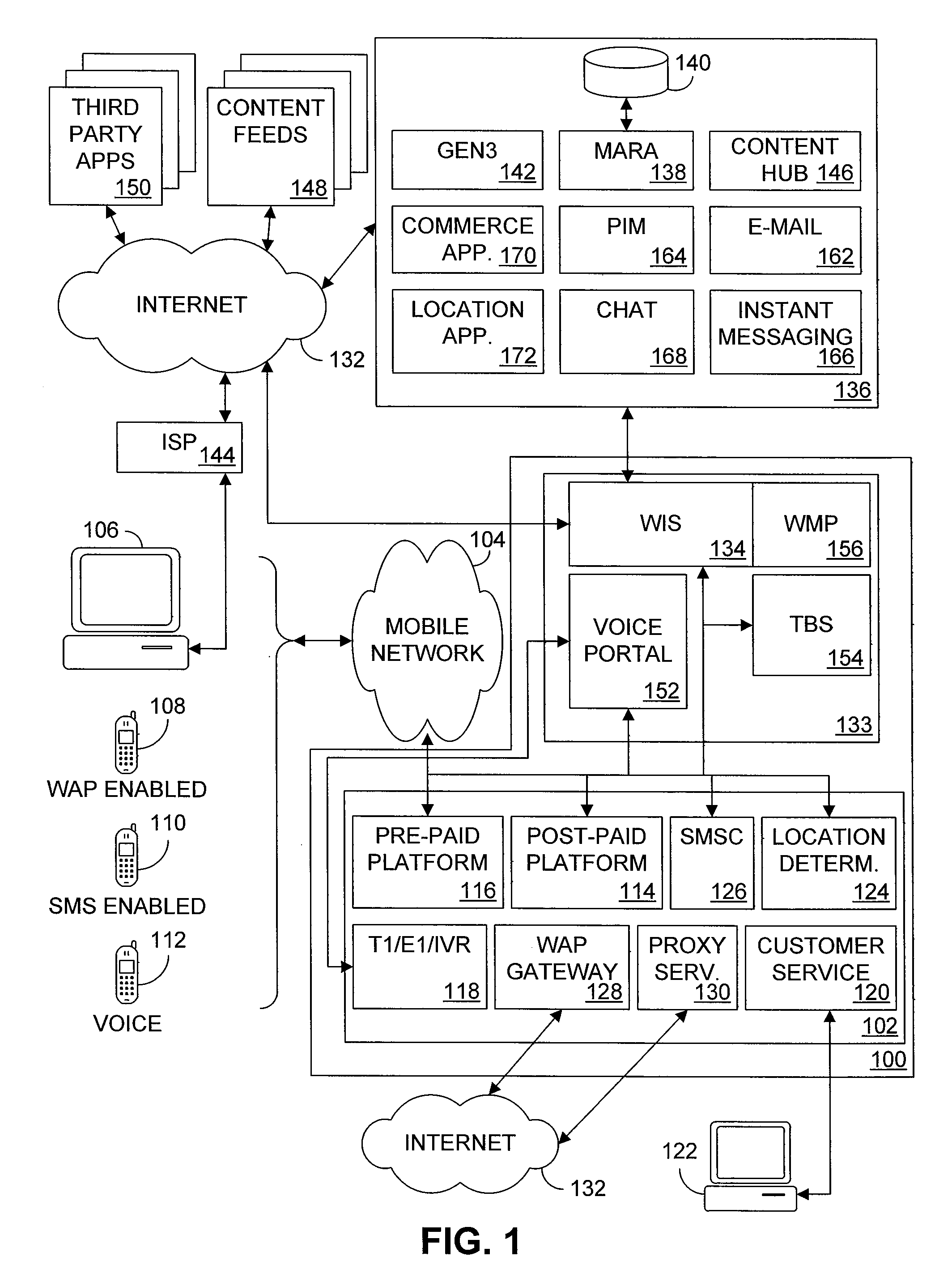
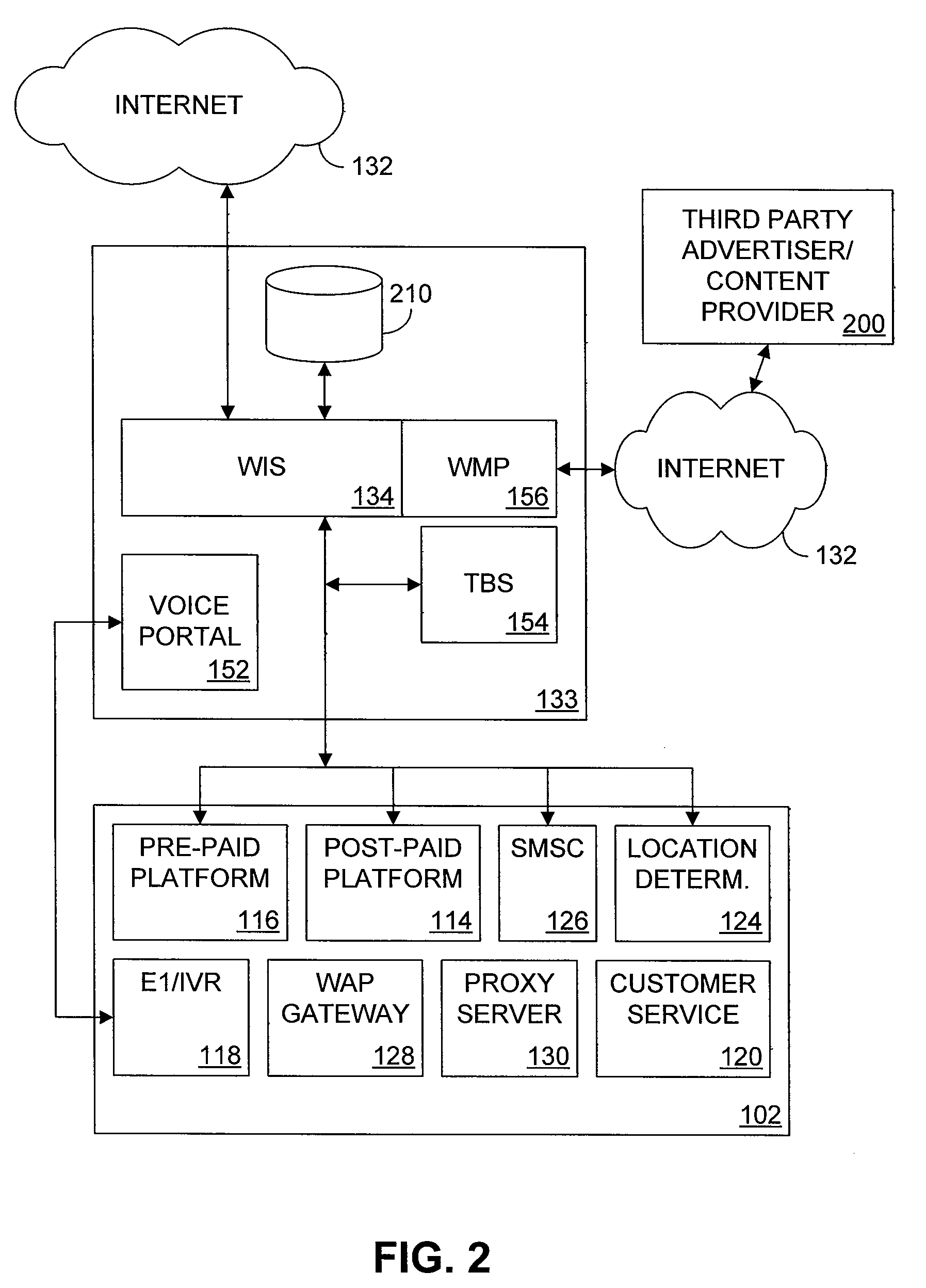
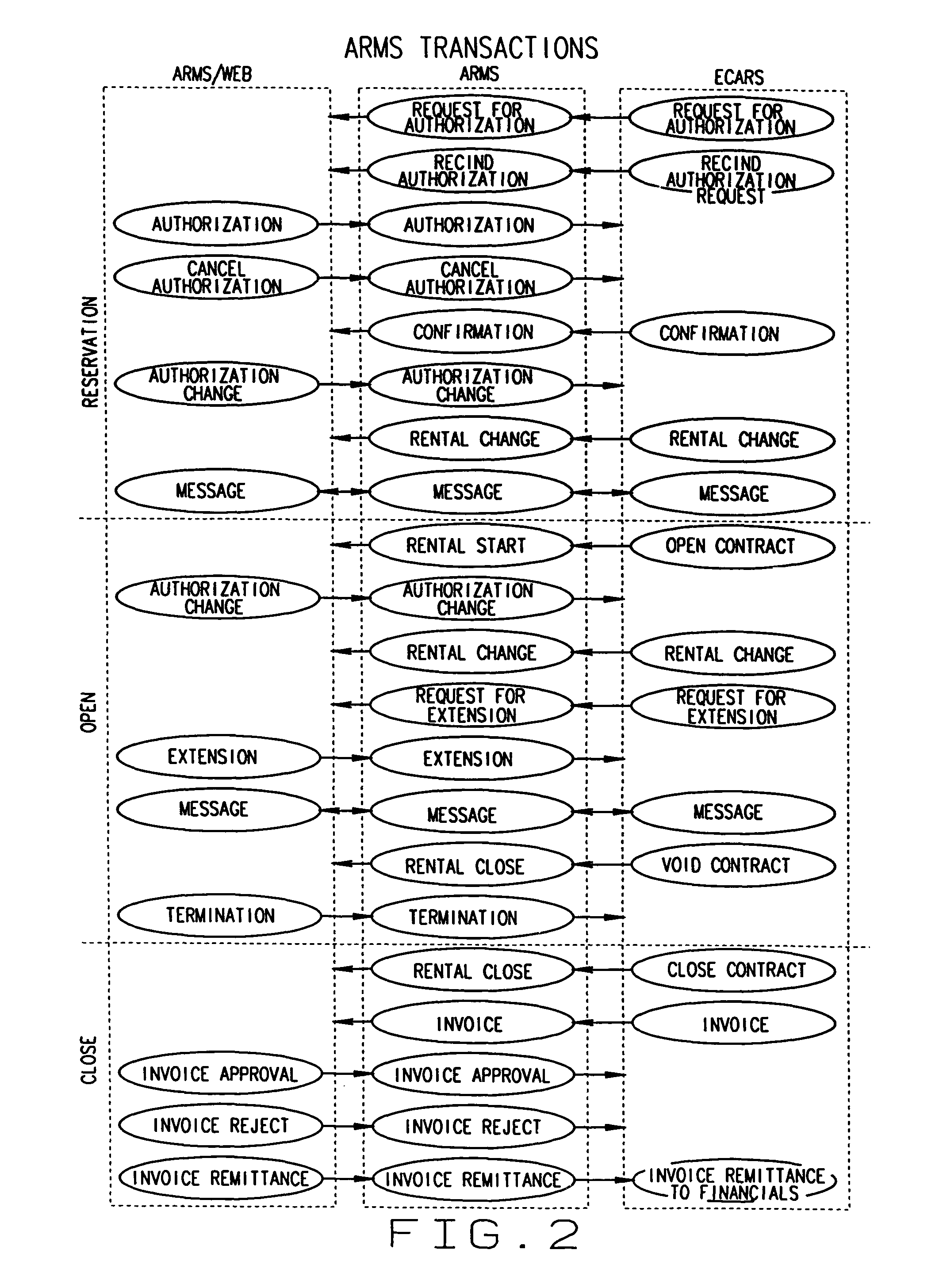
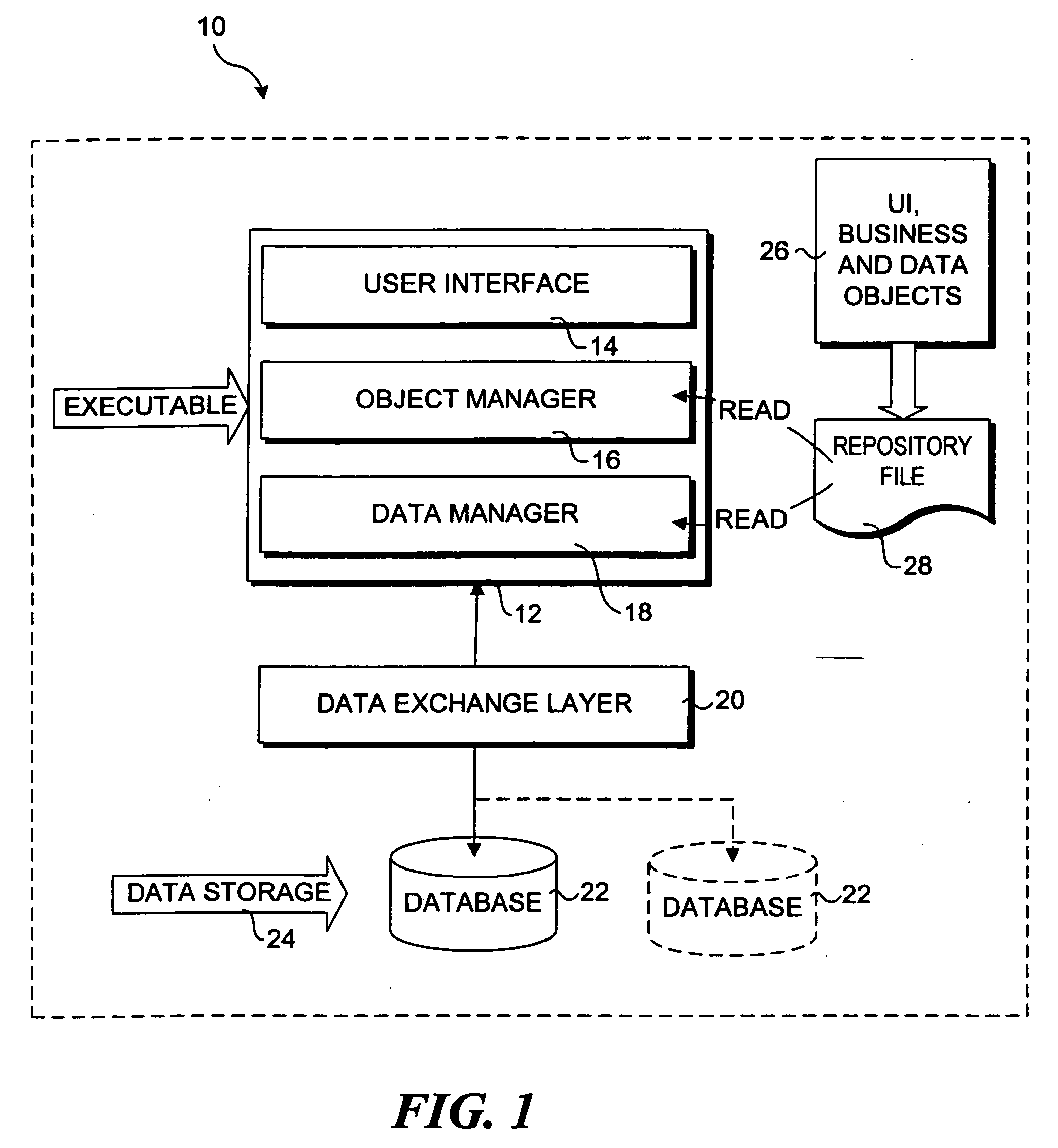
Multiple access internet portal revenue sharing
ActiveUS7127232B2Metering/charging/biilling arrangementsAccounting/billing servicesCable Internet accessInternet access
Multiple access internet portal billing systems are provided. A representative system, among others, includes a communication facility, a wireless internet server, and a transaction billing system. The communication facility includes a billing platform, and is operable to connect to a plurality of wireless device platforms through a mobile network, and to connect to the wireless internet server. The wireless internet server provides internet access to the wireless devices and communicate at least one billing information record including a usage time to a transaction billing system. The transaction billing system receives the billing information record from the wireless internet server, formats the billing information record, and communicates the formatted record to the communication facility billing platform. Methods and other systems for multiple access internet portals are also provided.
Owner:BELLSOUTH INTPROP COR
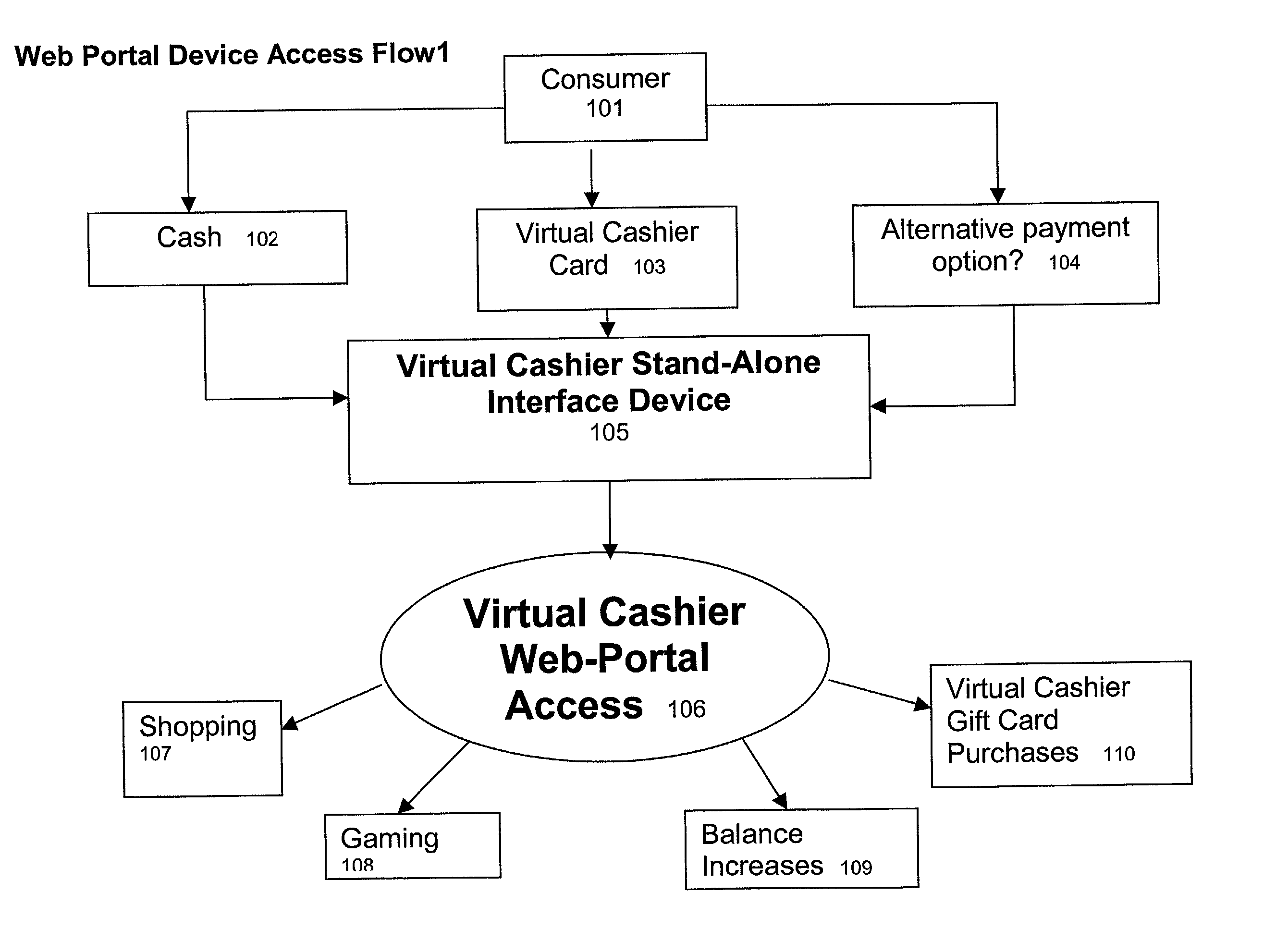
Virtual cashier
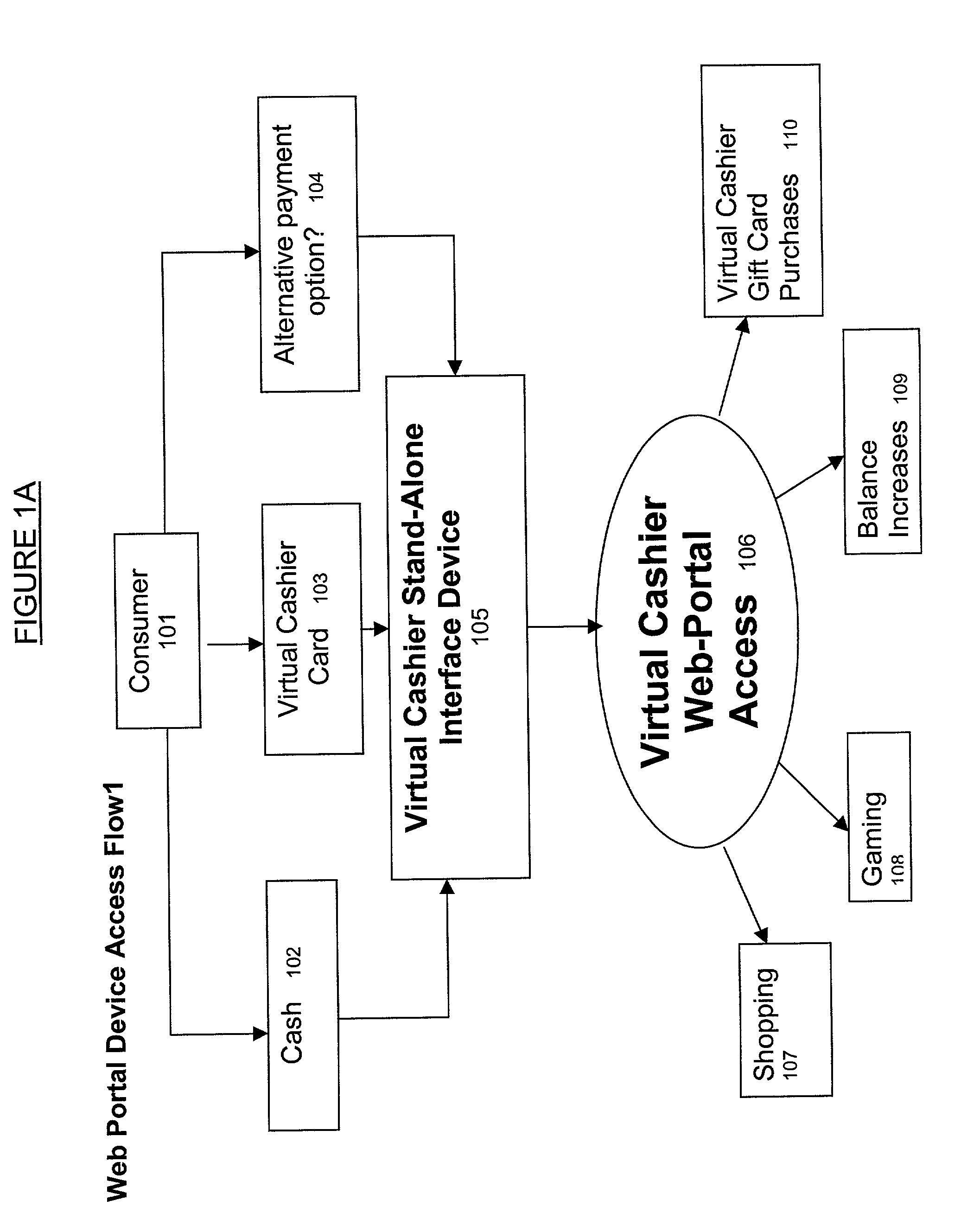
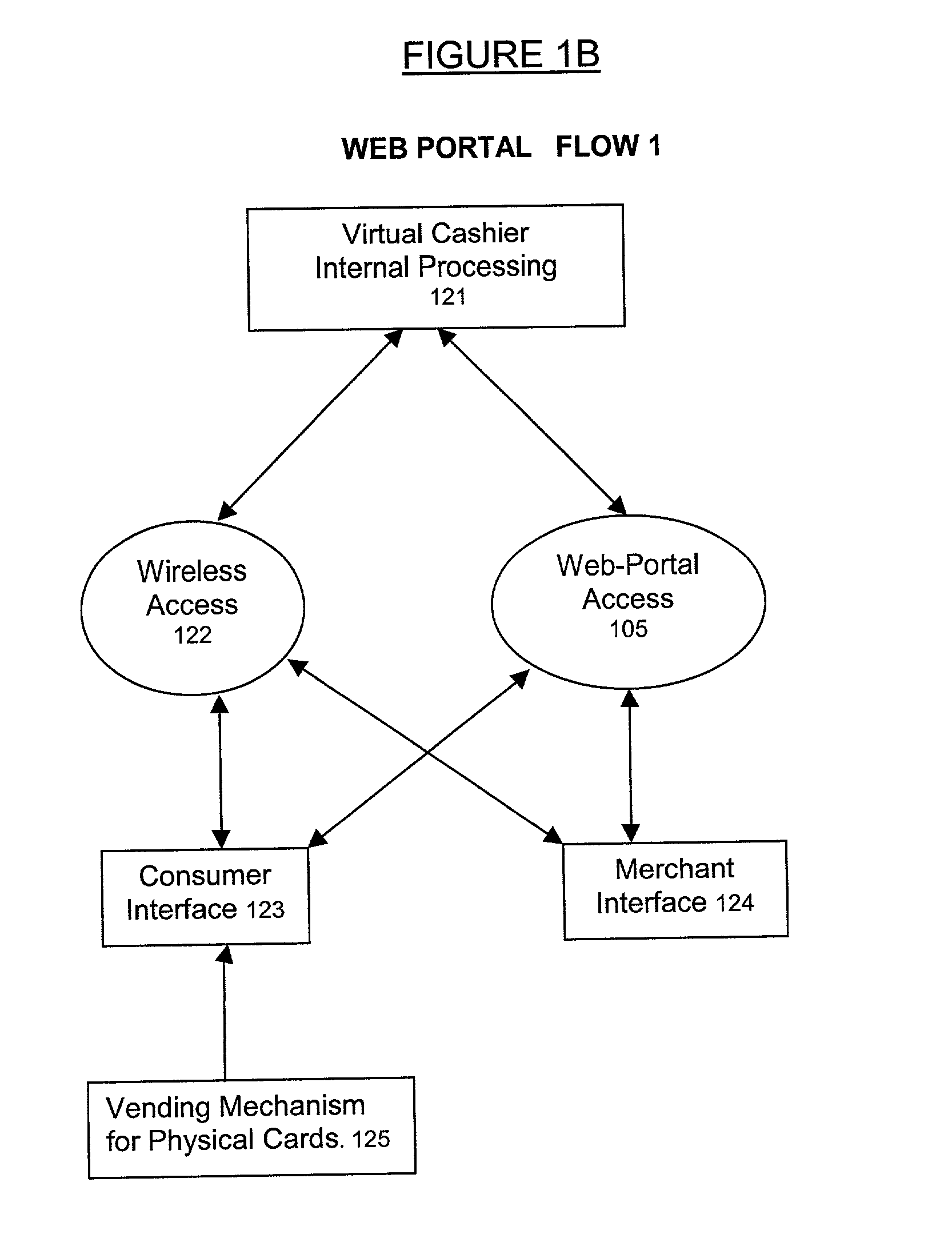
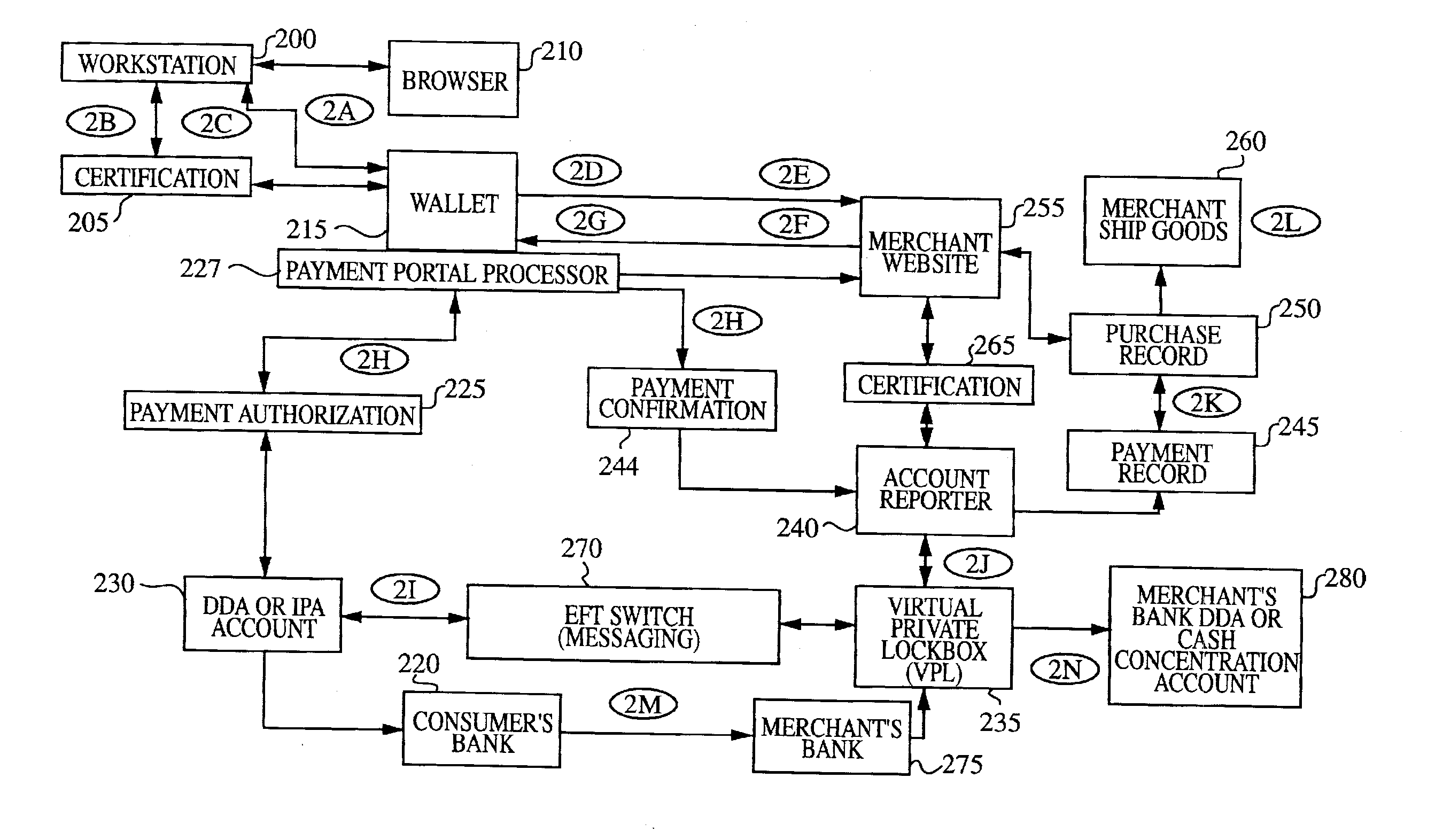
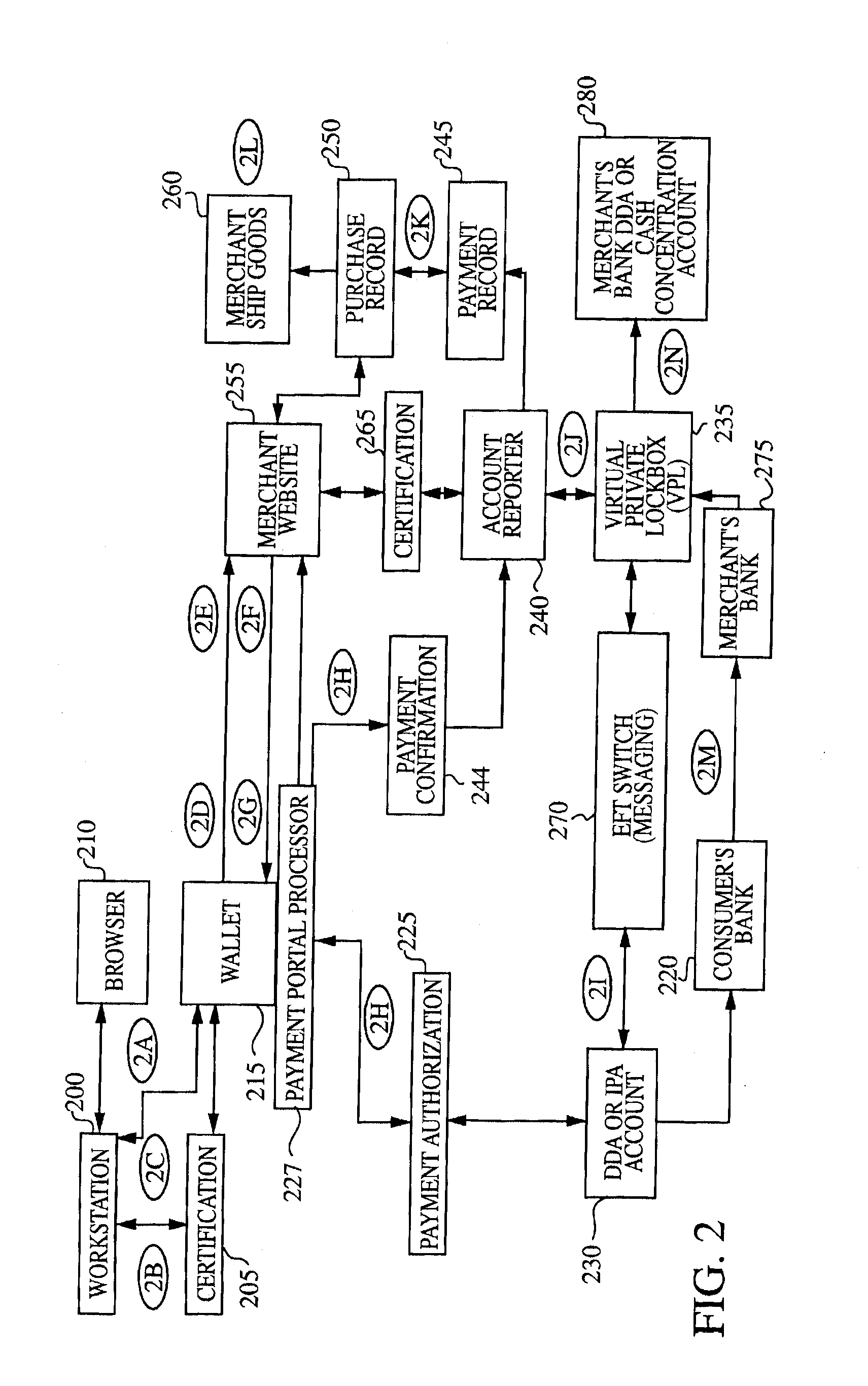
This invention pertains to a method and apparatus that prepaid cards or similar mechanisms to identify money accounts from which payment can be made to merchants and others via electronic transfer, particularly thorough communication transmitted over the Internet. The invention can allow electronic transfer of money without disclosure of individual identity or financial information. The apparatus can accept cash or other forms of payment that can be then transferred via an Internet site, permitting the merchant to receive payment directly from the entity maintaining the Internet site. The operator of the Internet site (Internet portal) will undertake collection and accounting of payments received from individual users or purchasers. The invention also comprises an apparatus that can be utilized to provide Internet access to the public similar to a public telephone and ability to conduct financial transactions over the Internet similar to an ATM.
Owner:MICRODEVERSITY
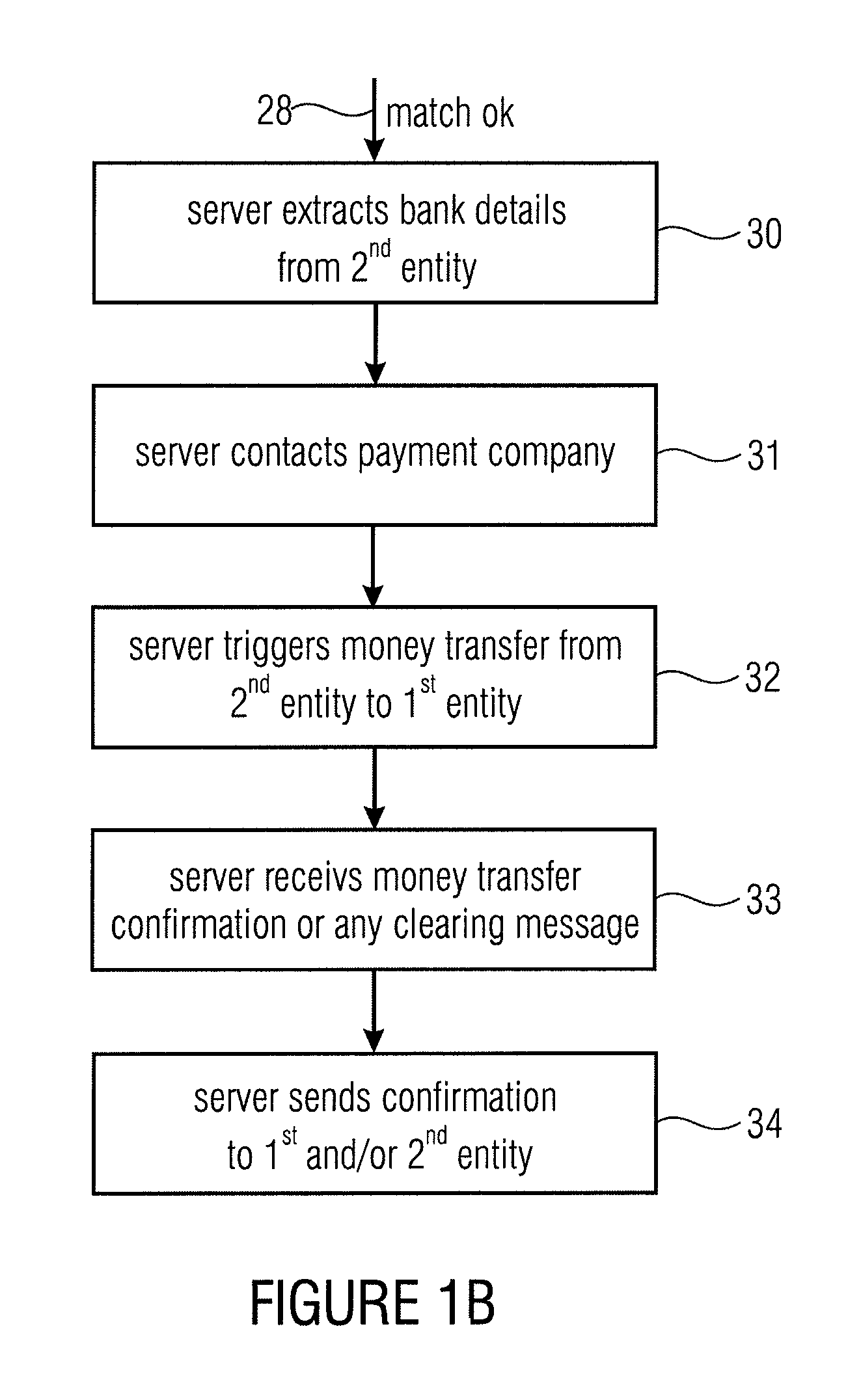
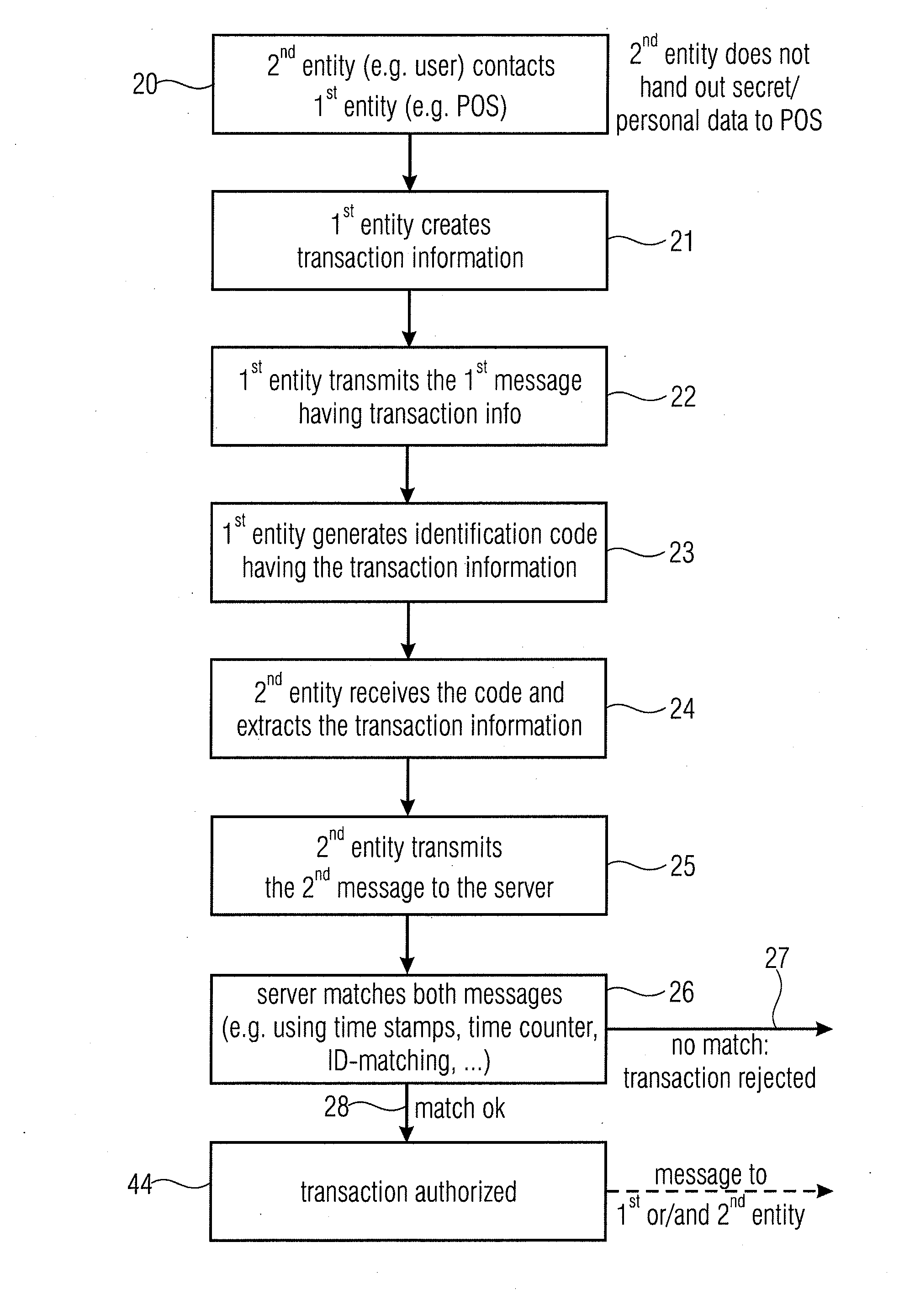
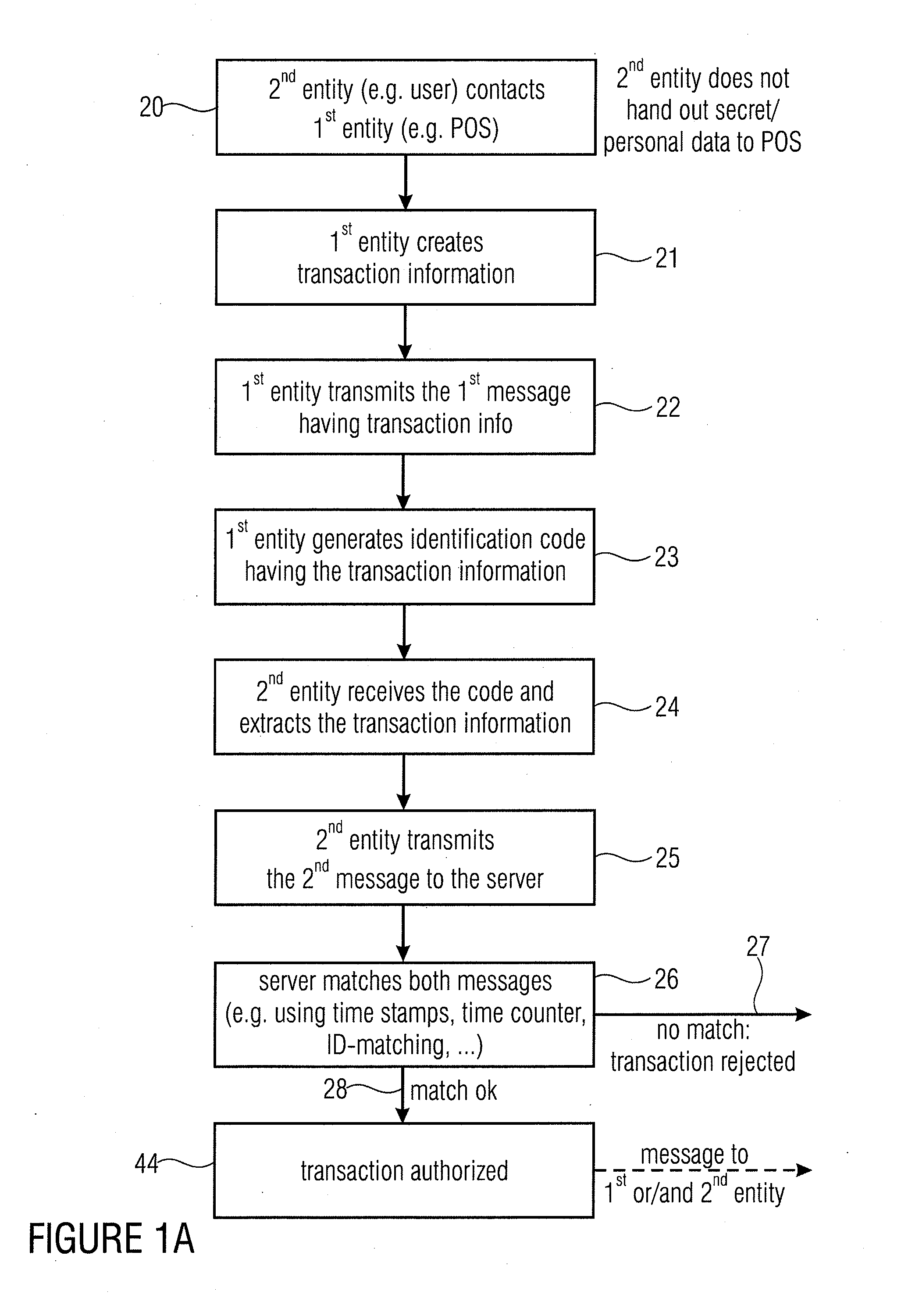
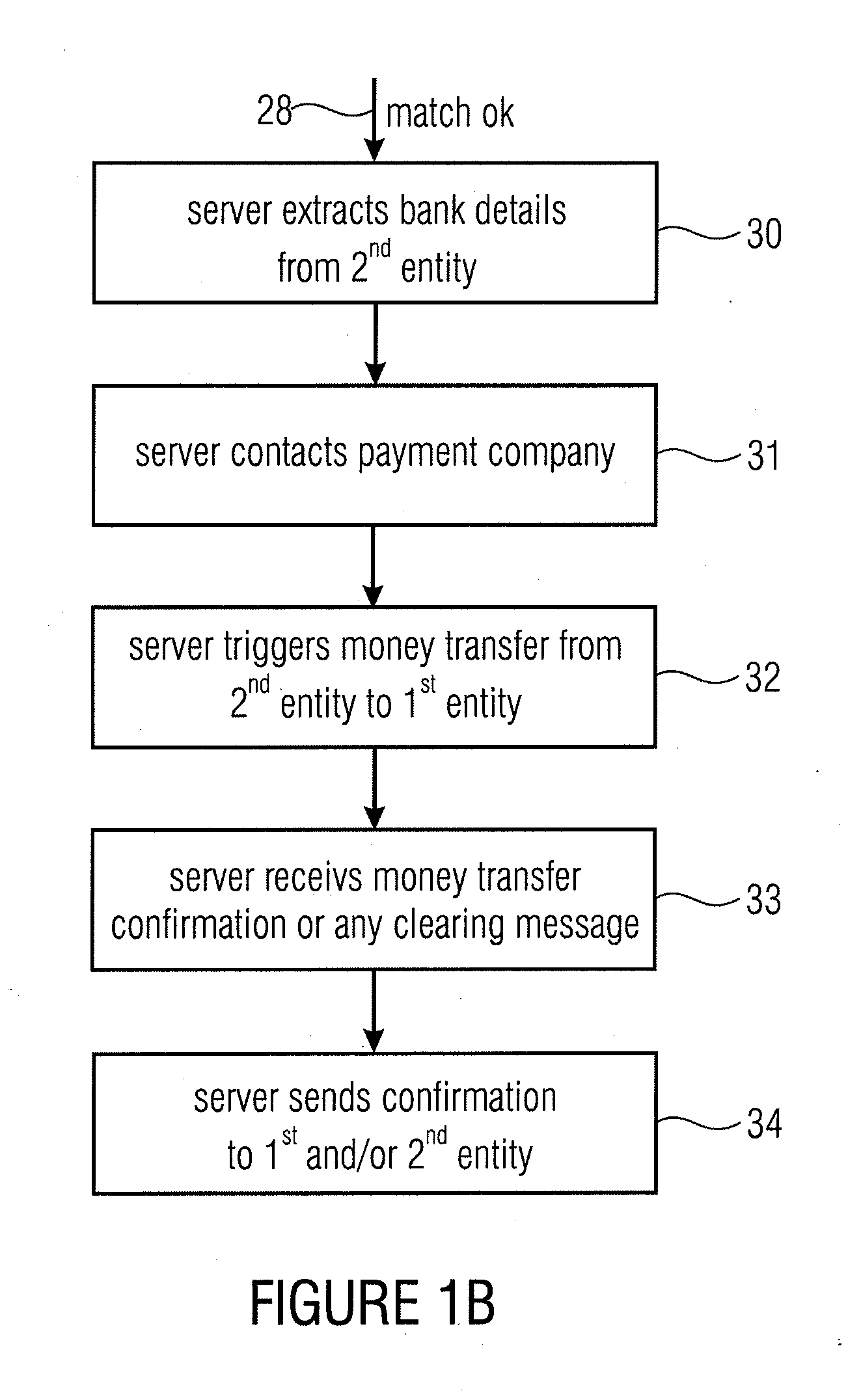
Server device for controlling a transaction, first entity and second entity
InactiveUS8332323B2Convenient security checkContributes to the convenience for the userComplete banking machinesFinancePaymentServer appliance
Owner:MR QR10
Server Device for Controlling a Transaction, First Entity and Second Entity
InactiveUS20110137797A1Convenient security checkContributes to the convenience for the userComplete banking machinesFinancePaymentServer appliance
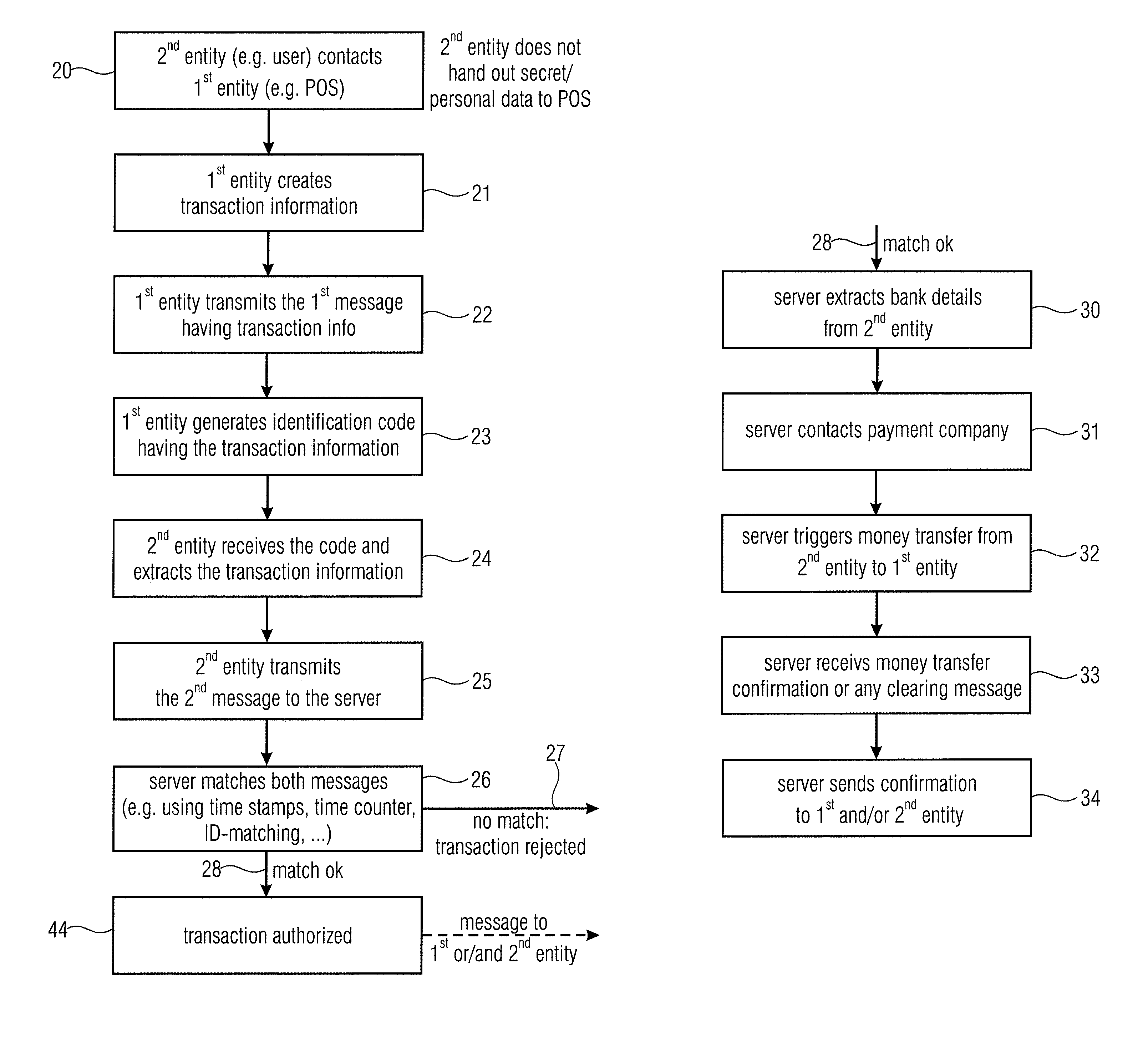
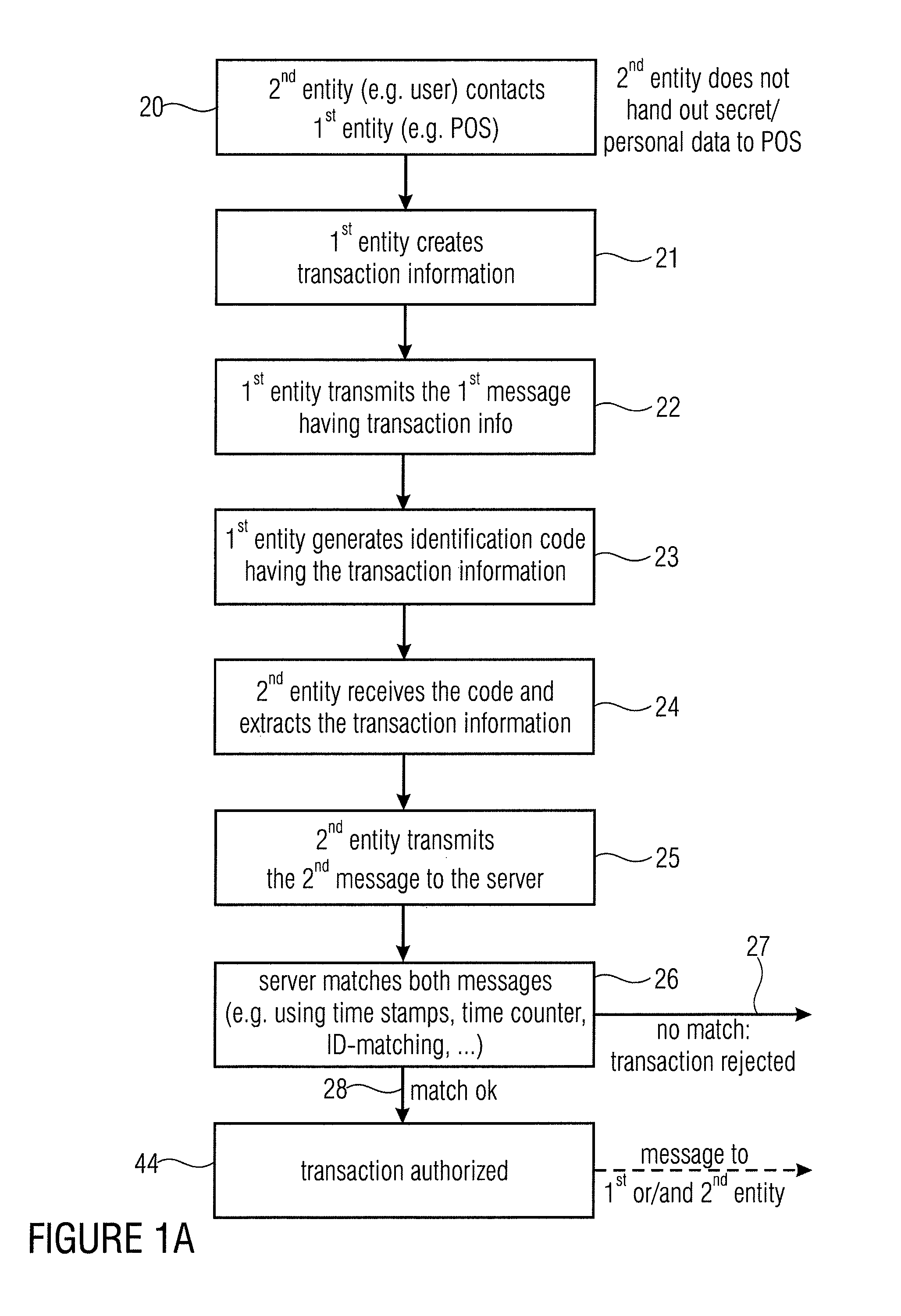
Server device for performing a transaction in a system having a first entity, such as a POS, a second entity, such as a user having a mobile phone with a digital camera, and a remote server. The first entity generates a code having a transaction information and sends a first message to a server. The second entity, such as a buyer of a product or a user of a service captures the code and transmits a second message to the server having information on the transaction extracted from the code. The transaction is only authorized, when the server has determined that the first message and the second message match with each other. The transaction can be a payment transfer, a grant of an access to a service or a grant of an access to an internet portal.
Owner:MR QR10
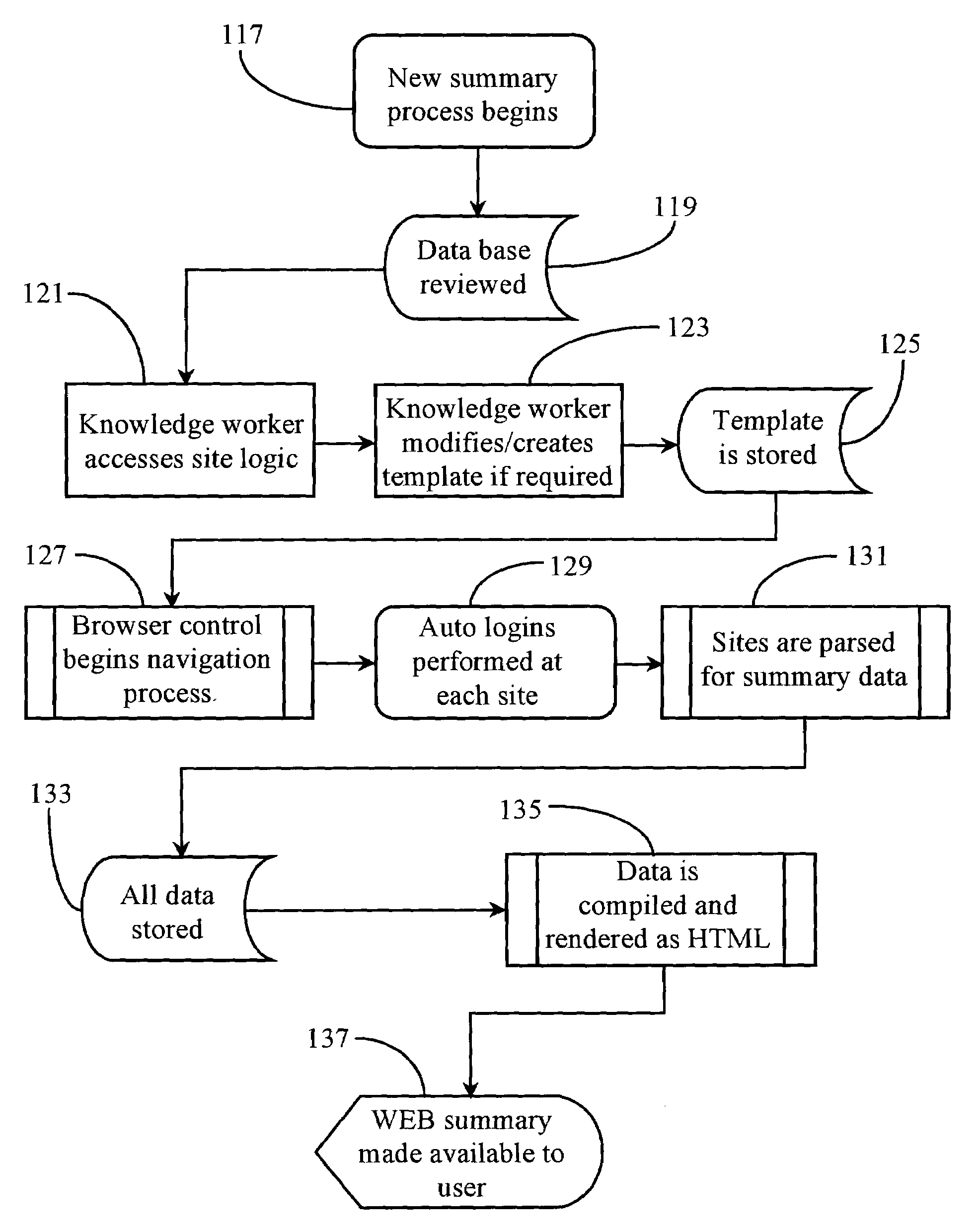
Network-based bookmark management and web-summary system
InactiveUS7085997B1Digital data information retrievalDigital computer detailsUniform resource locatorClient-side
A network-based URL management and data gathering system is provided. The system utilizes a client-side utility for capturing URLs during normal Web browsing, and a server-side utility for organizing and managing the captured URLs on the network. The server-side utility periodically sends a request to a proxy browsing and data gathering utility for navigating to and retrieving data from Web pages associated with the captured URLs. Data retrieved from the Web pages is returned in summary form for presentation to subscribing users. In preferred embodiments, the system is practiced on the Internet network between users operating an Internet-capable appliance having an Internet connection, and an Internet portal service.
Owner:YODLEE COM INC
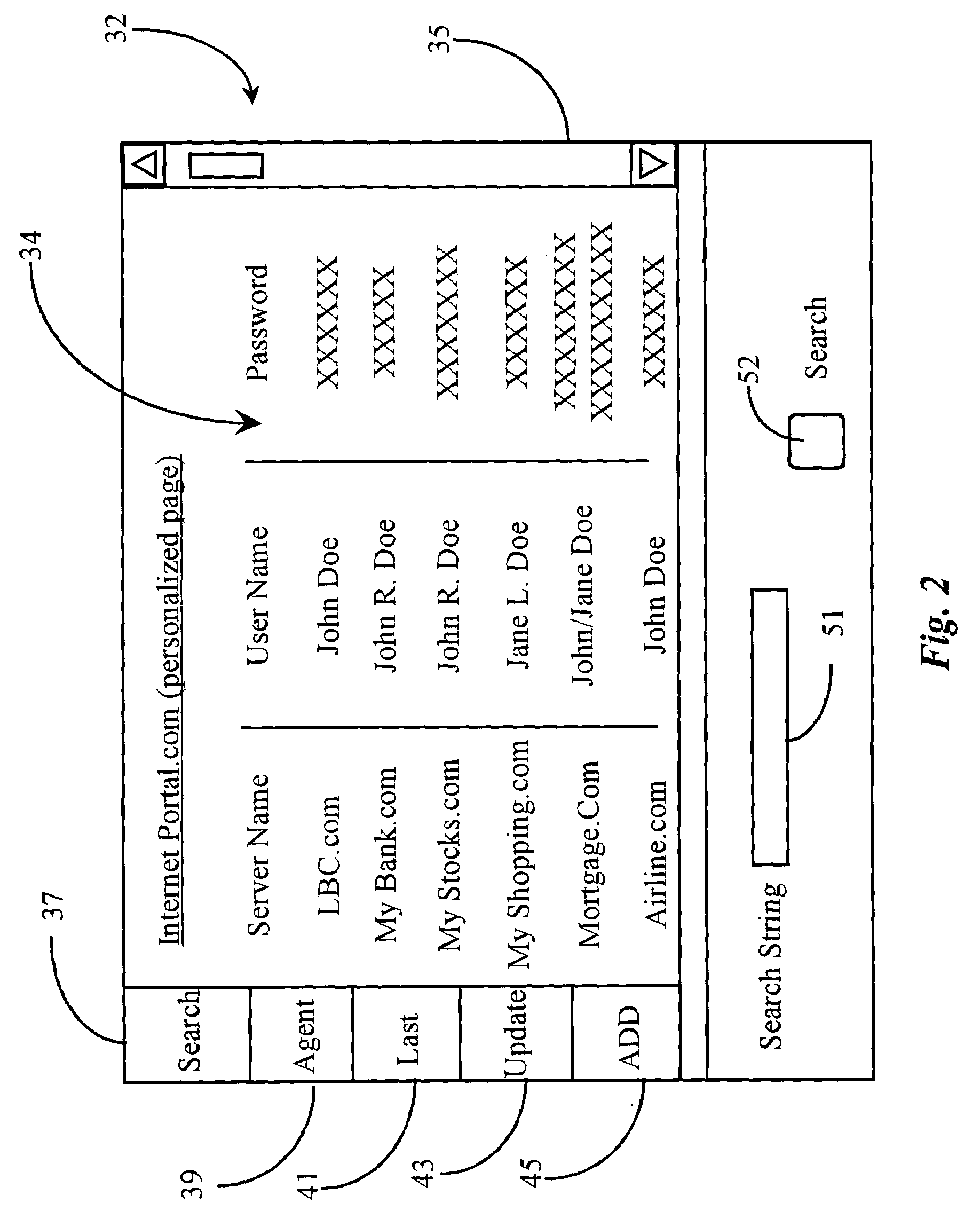
System and method for customized portal web pages
InactiveUS20050097190A1Easy to customizeIncrease volumeMultiple digital computer combinationsWeb data navigationWeb siteWeb page
A system and method for providing a personal Internet portal page to a user that displays at least some content selected by the user from a different Internet web site. The personal Internet portal page of the present invention displays at least some content from by the user. Moreover, the present invention provides a personal Internet portal page that displays only that content which is selected by the user. The present invention provides an Internet portal that obtains a live feed of data from one or more of servers. The data are provided in accordance with predefined criteria and presented in one or more encapsulated formats. The formats can be customized to accommodate individual preferences.
Owner:ABDELHAK AARON
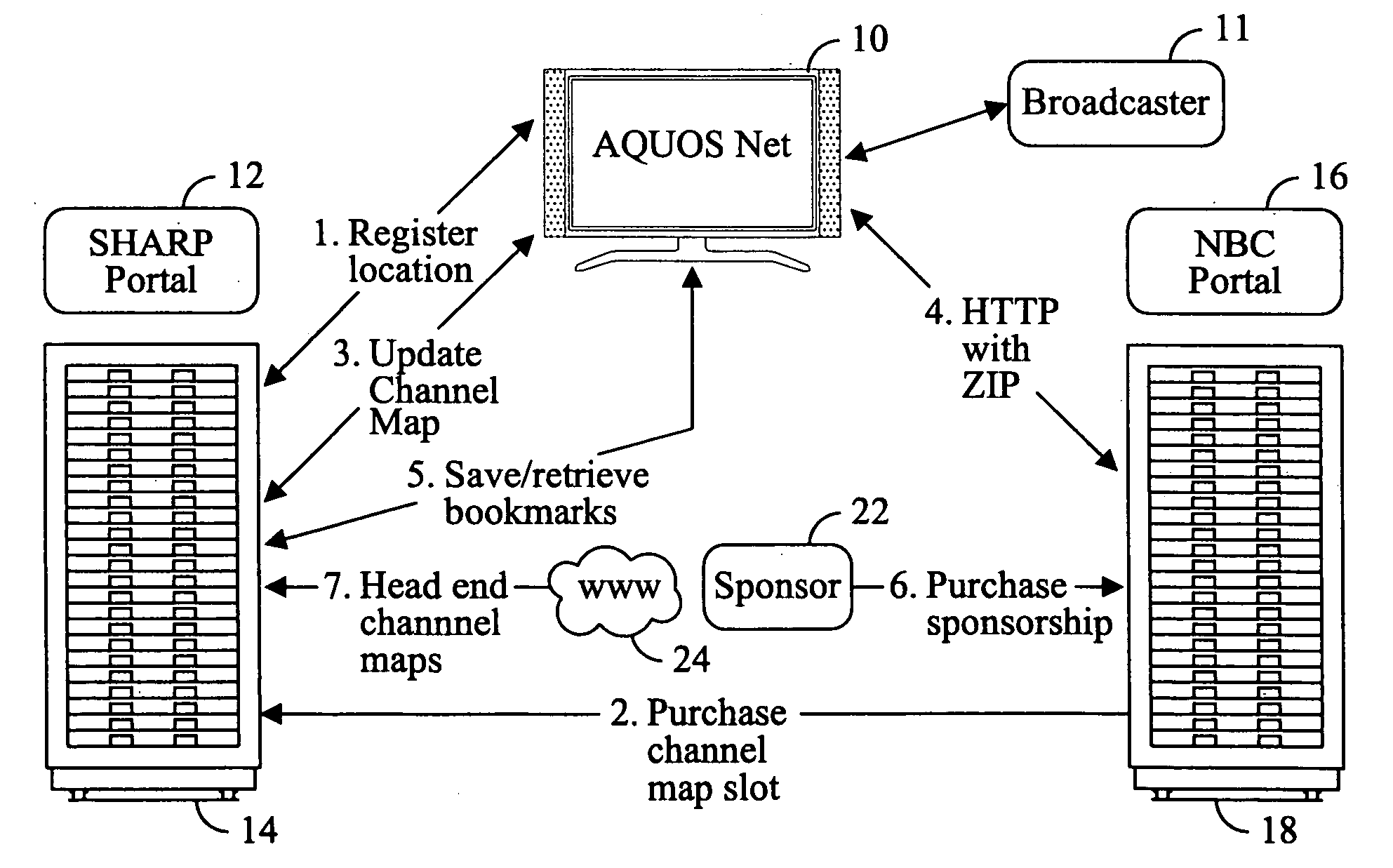
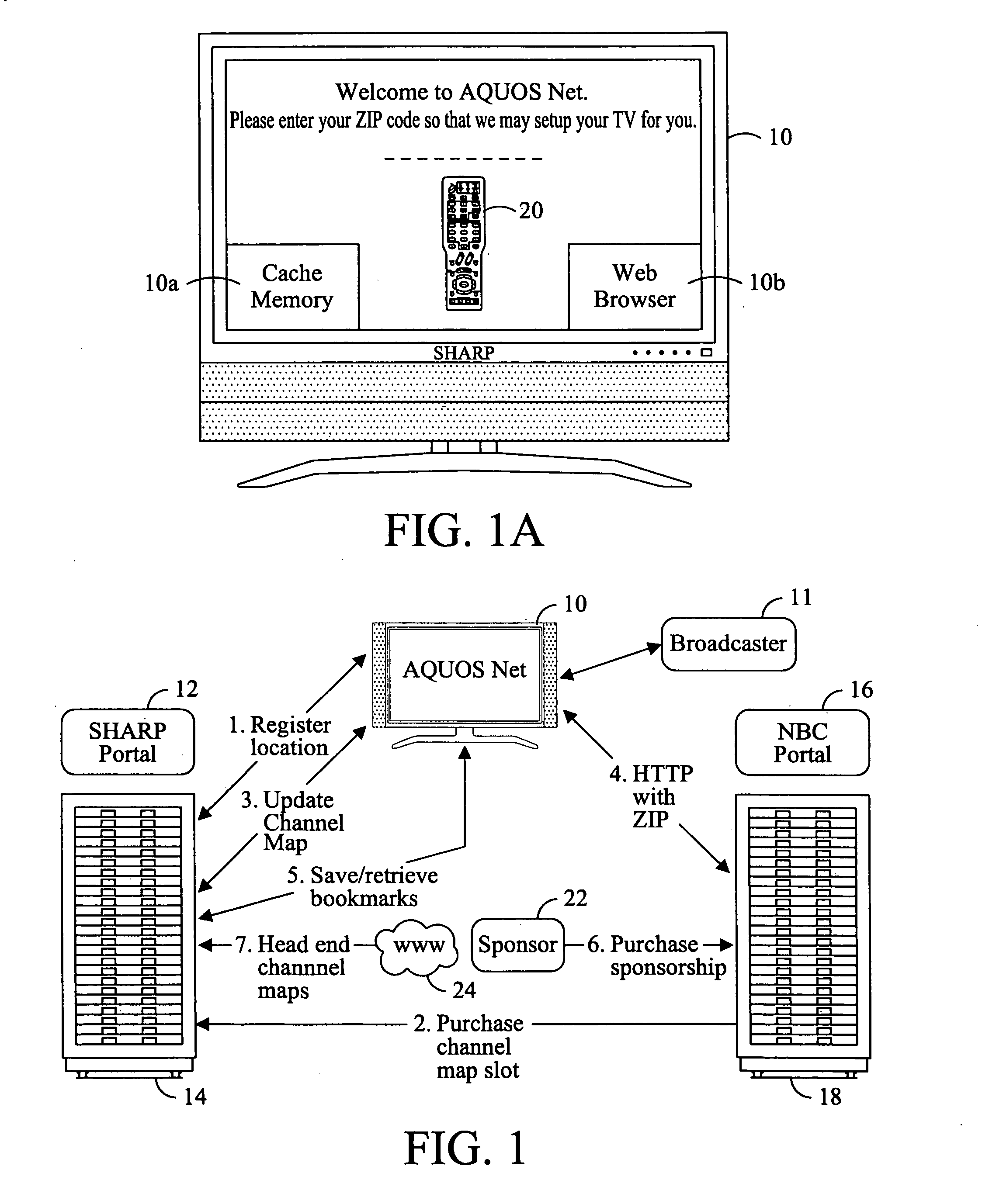
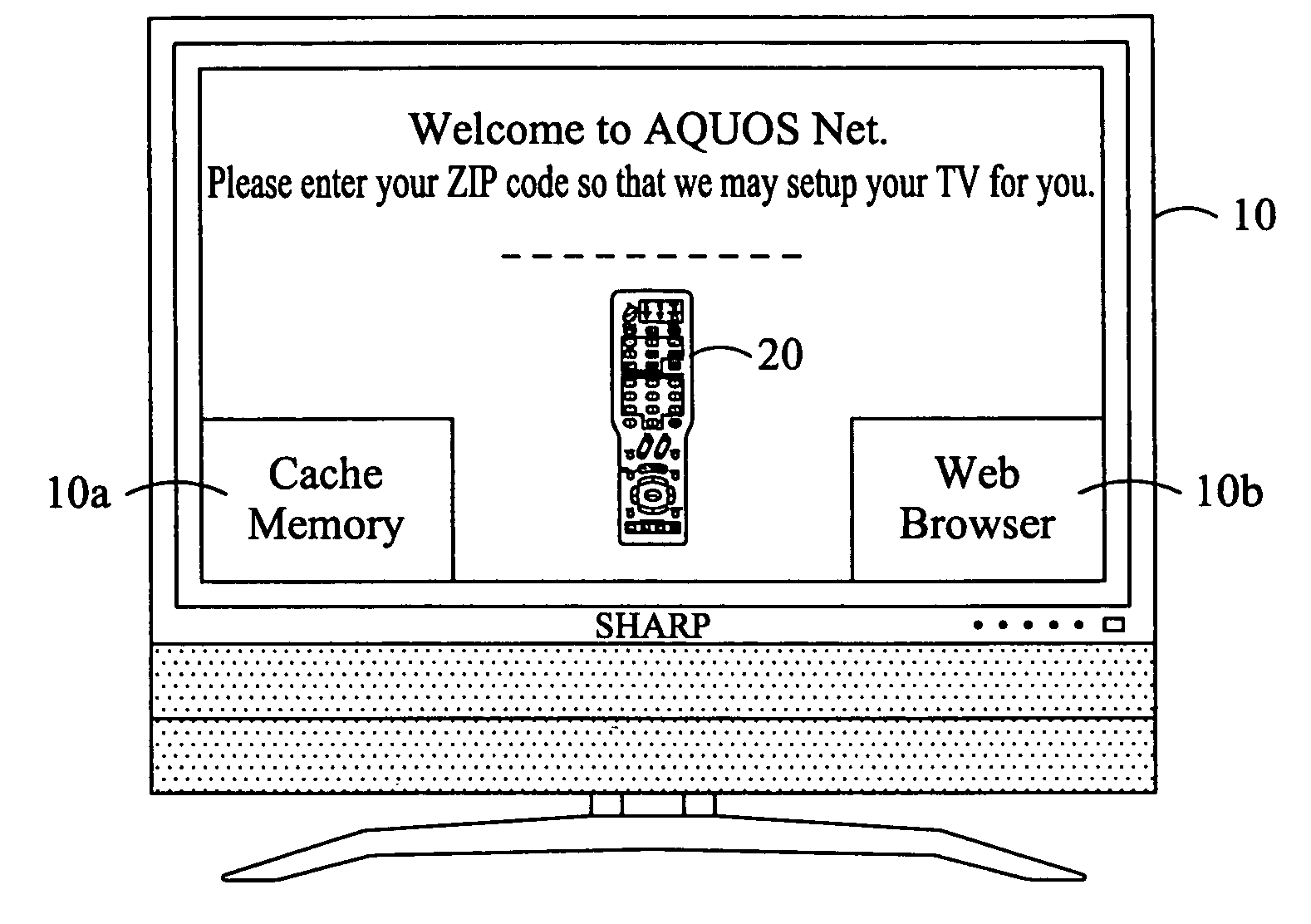
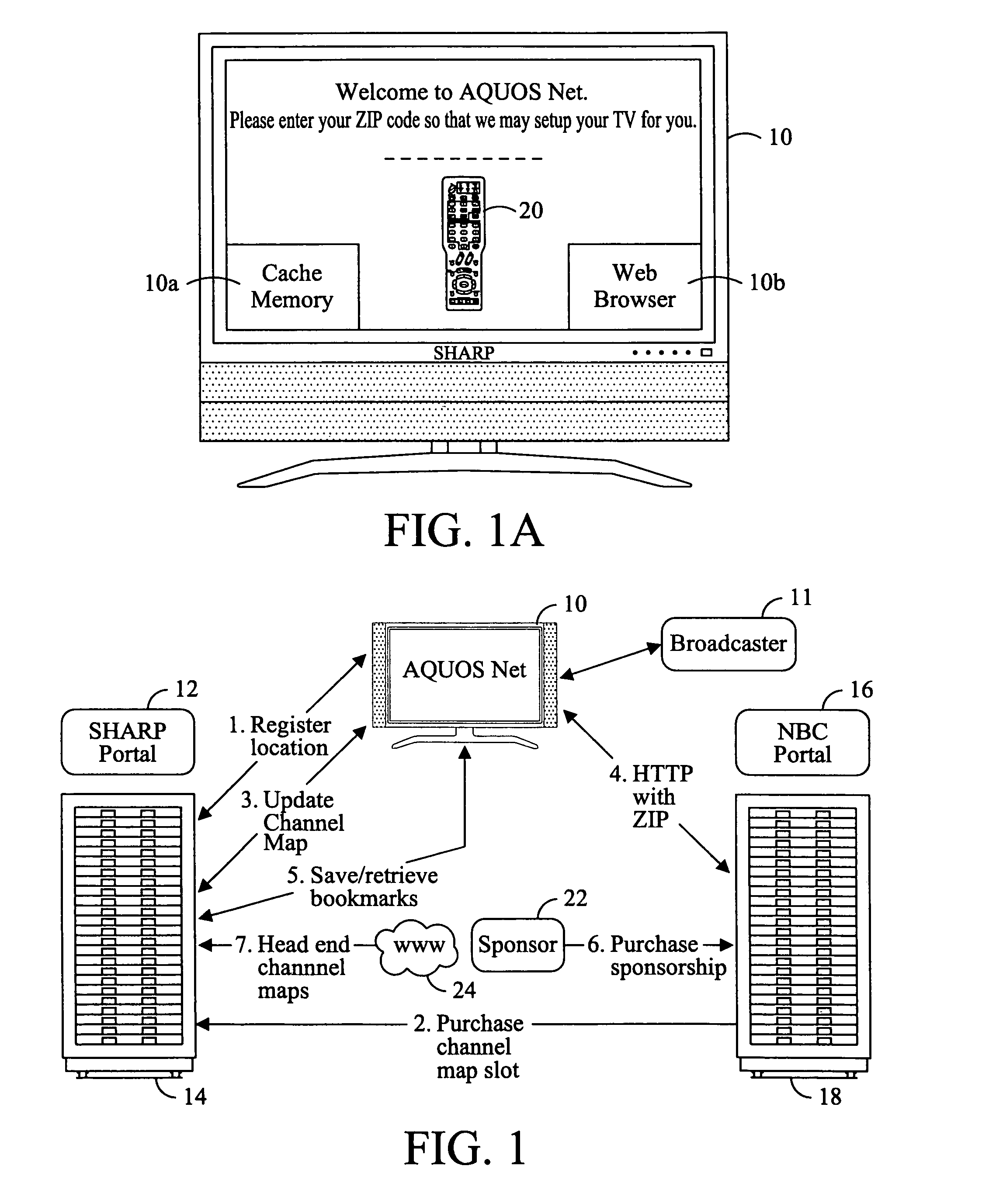
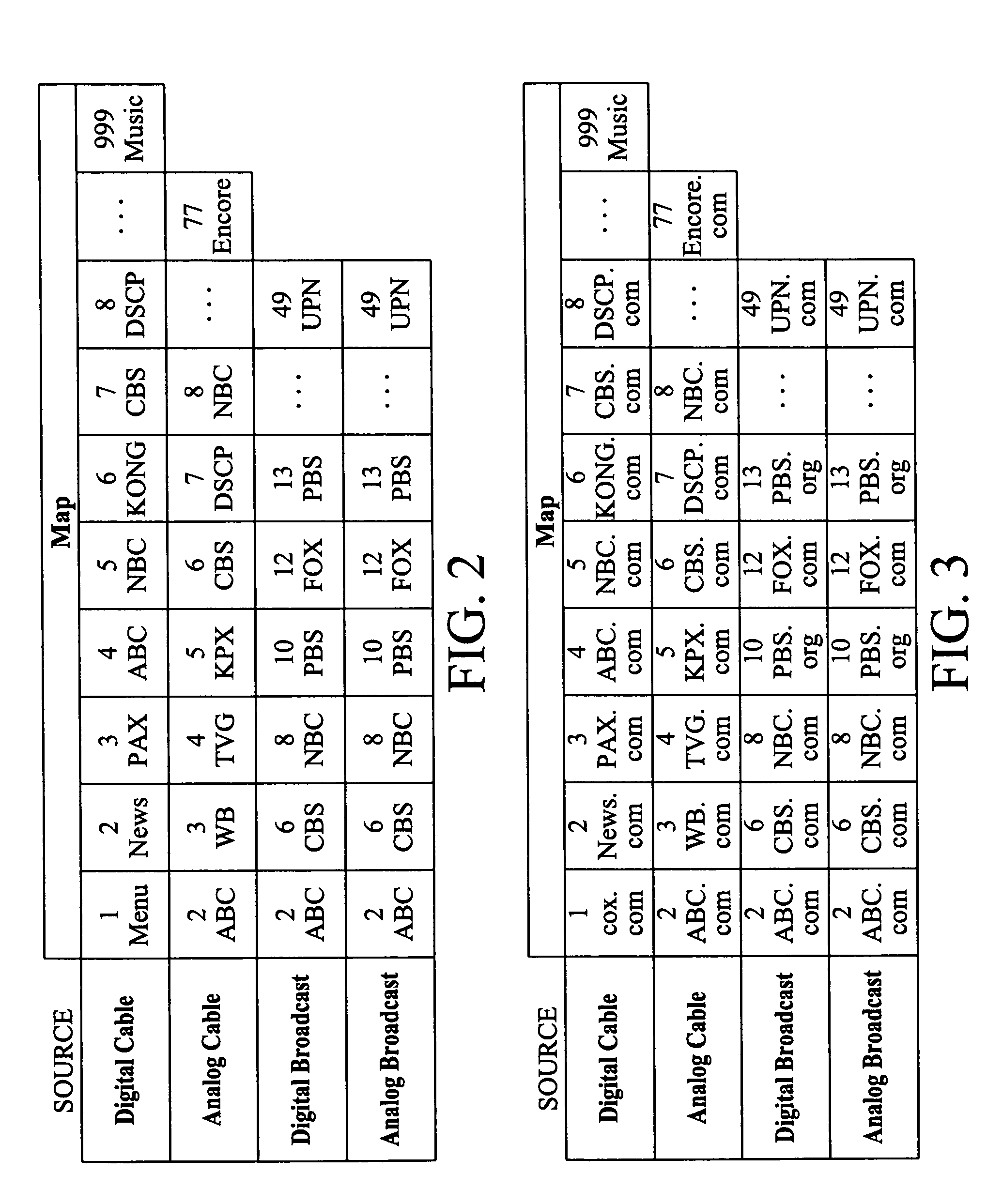
Television system having internet web browsing capability
InactiveUS20080046945A1Two-way working systemsSelective content distributionTelevision systemWeb browser
A television system for providing standard television programming and Internet browsing includes a television set for viewing digital and / or analog programming and includes a memory storage device and an integral embedded web browser for displaying data received from an Internet portal. The data is in the form of URLs and web pages that are synchronized with selected TV programming and / or advertising. A remote control selectively displays URLs and / or their associated web pages stored in memory by the Internet portal. In one aspect of the invention, TV programming can be displayed simultaneously on the screen with related web pages and URLs.
Owner:SHARP KK
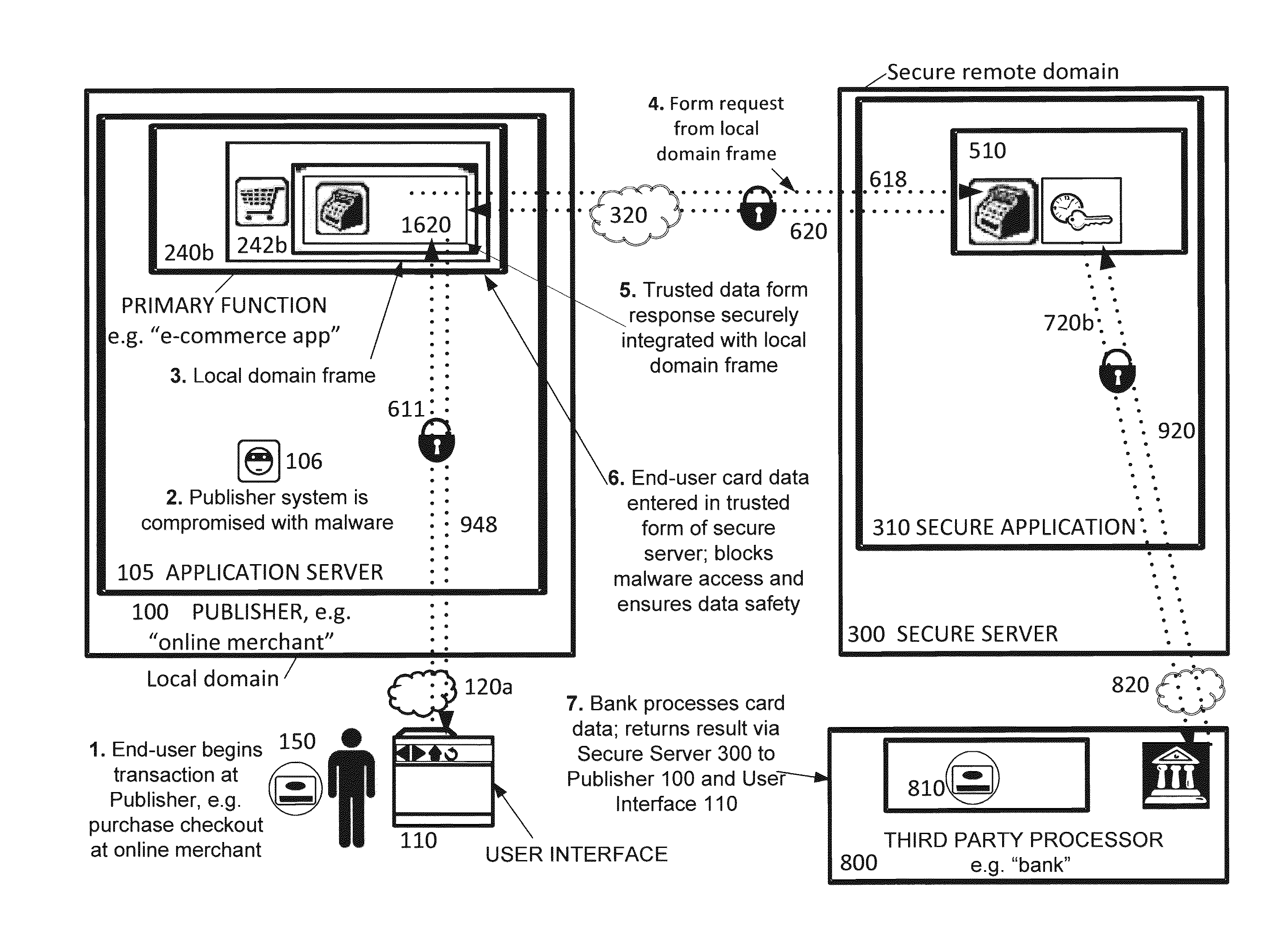
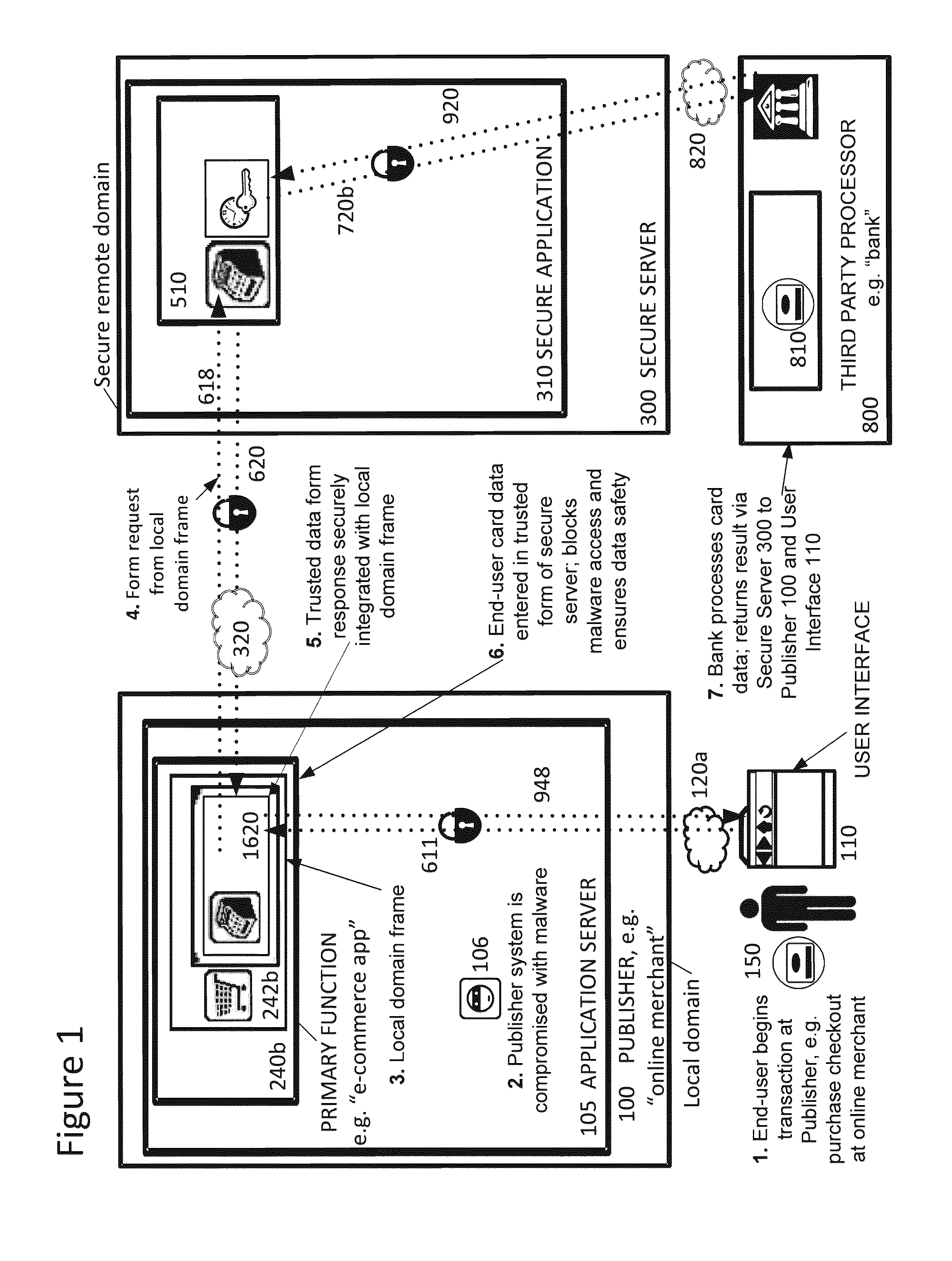
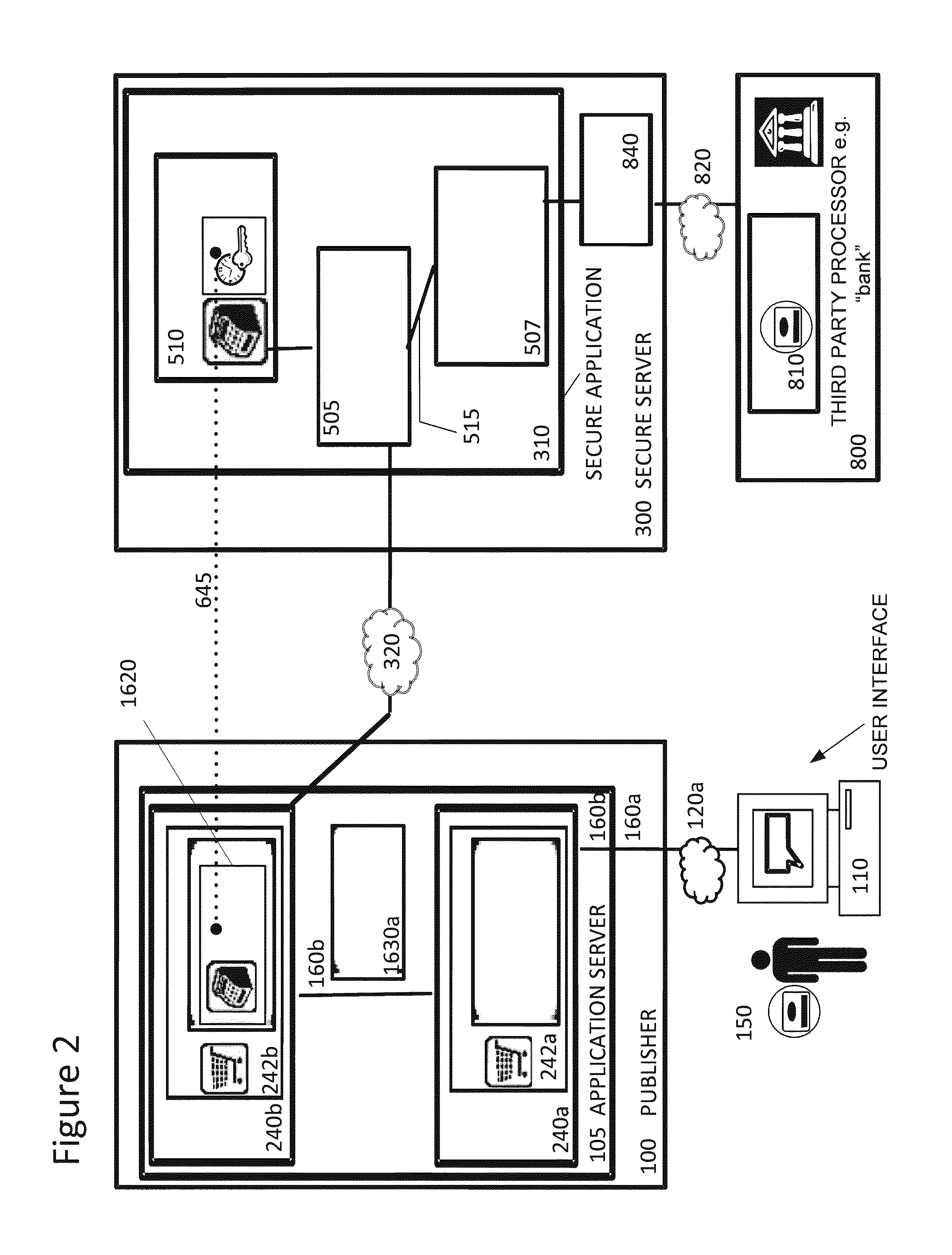
Securing sensitive information with a trusted proxy frame
InactiveUS20120089481A1Rapid deploymentInformation securityDigital data processing detailsUser identity/authority verificationThird partyInternet privacy
A system and method for secure transmission of sensitive end user information from an Internet portal operated by a publisher to a third party data processor. The publisher provides a content portal such as an e-commerce or healthcare information site. A third party data processor such as a bank or healthcare organization requires the sensitive information for a data processing function. In response to the requirement for sensitive information, a trusted proxy frame is invoked from a secure server operative to securely communicate the sensitive information. The trusted proxy frame is displayed in a secure context in the end user's browser and receives input of the sensitive information. The sensitive information is encrypted and communicated through the secure server to the third party data processor. Results of this processing are transmitted to the publisher through a novel callback process that enables the publisher to execute its data processing functions, as if it was in possession of the sensitive information, but without actual access to the sensitive information. The third party data processor returns an acknowledgement of processing of the sensitive information.
Owner:CHAIN REACTION ECOMMERCE
Method and system for processing internet payments using the electronic funds transfer network
InactiveUS20130317984A1Safe, sound, and secureFinanceAnonymous user systemsFinancial transactionNetwork processing
Embodiments of the invention include a method and system for conducting financial transactions over a payment network. The method may include associating a payment address of an account with an account holder name, the account residing at a financial institution and the associated payment address of the account configured to allow withdrawals by the account holder only and to allow a plurality of deposits to be made at different times. The method further includes freely publishing the payment address and making it available to users of an internet portal or search engine. The method further includes receiving data over a network identifying a deposit to be made to the account, assigning the deposit to the account using the payment address, and notifying the payer of the assignment. At least one directory is used for associating the account holder with the payment address.
Owner:JPMORGAN CHASE BANK NA
System for inexpensively executing online purchases
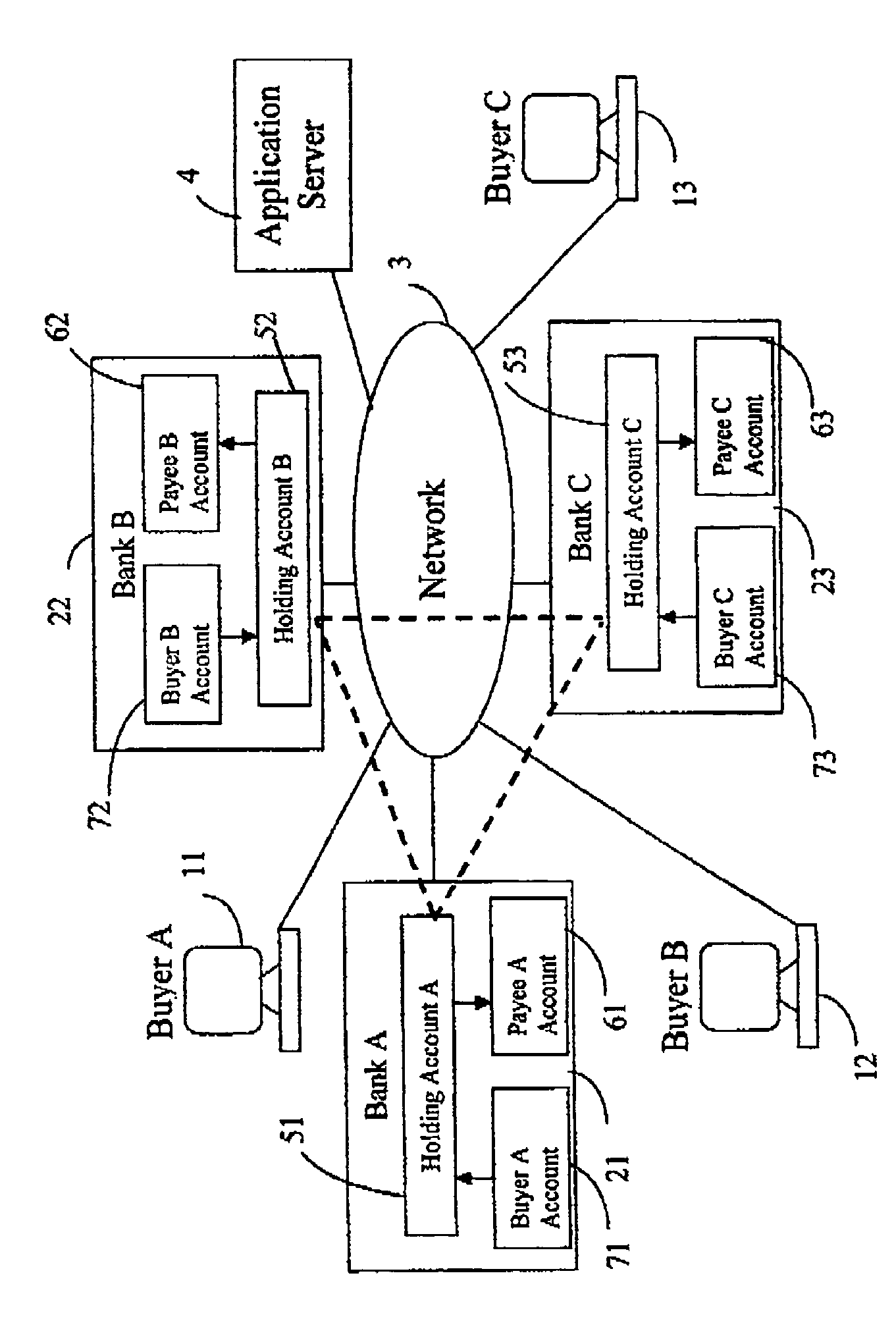
InactiveUS7092913B2Speeds the execution of online transactionsFinanceMultiple digital computer combinationsThird partyBank account
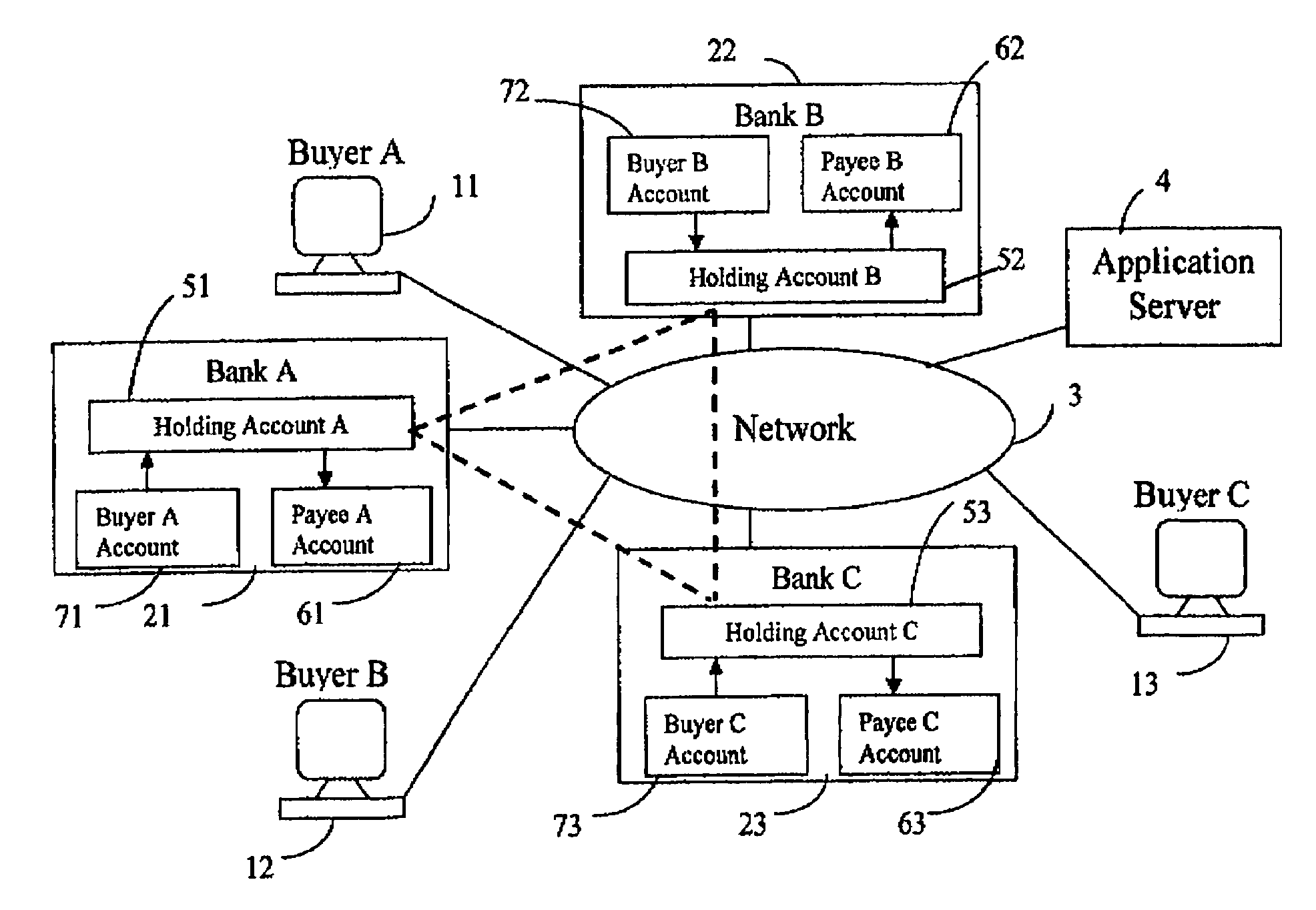
The present invention is a system for making online purchases using electronic funds transfers from the buyers' bank account to the vendor's bank account, enabled by an intermediate funds transfer from the buyer's account to a holding account maintained by the buyer's bank or a third party. The system is further enabled by the buyer's bank acting as a portal to the Internet that pre-authenticates buyers, enforces security, and speeds the execution of online transactions.
Owner:CANNON JR THOMAS CALVIN
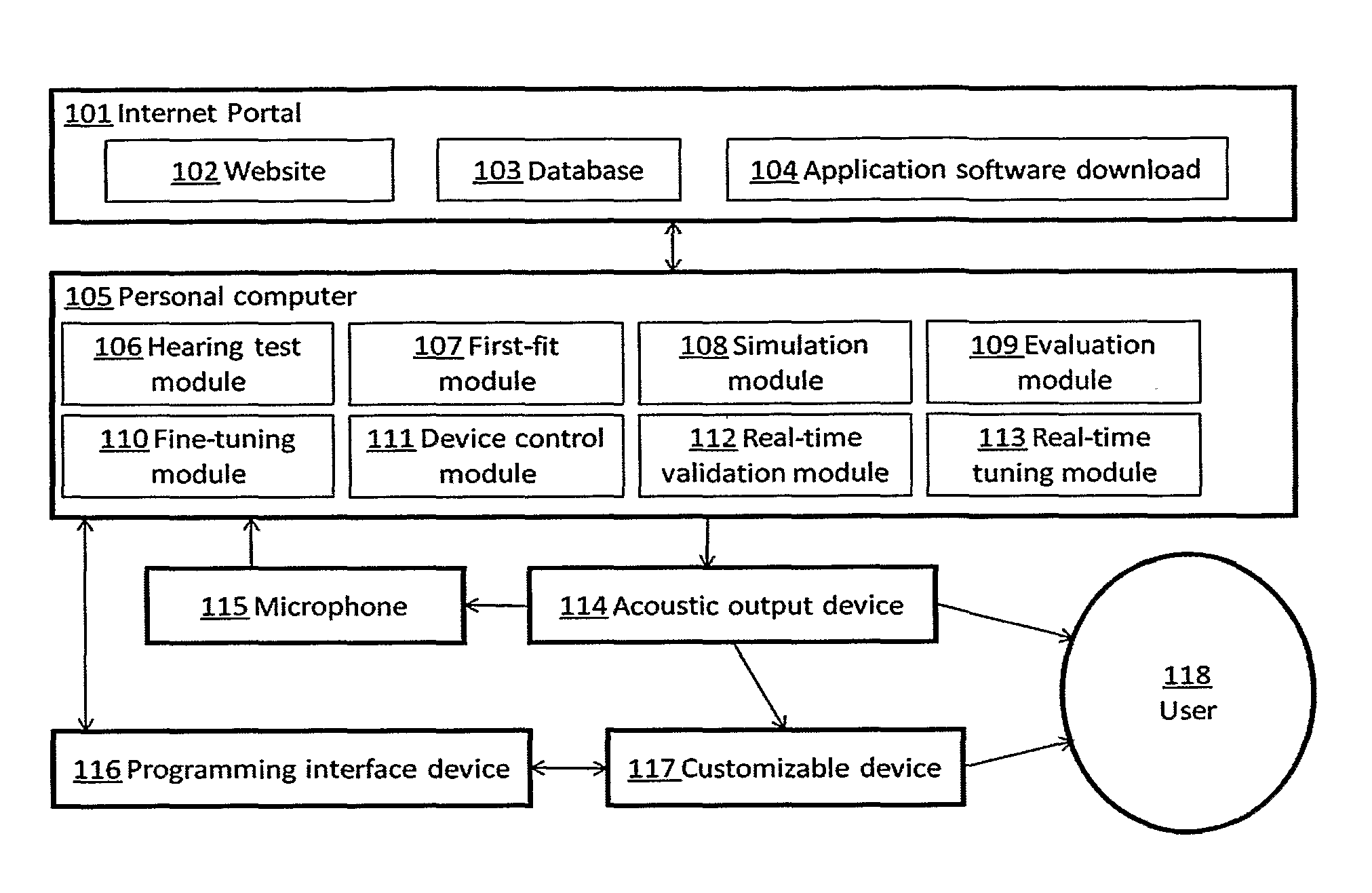
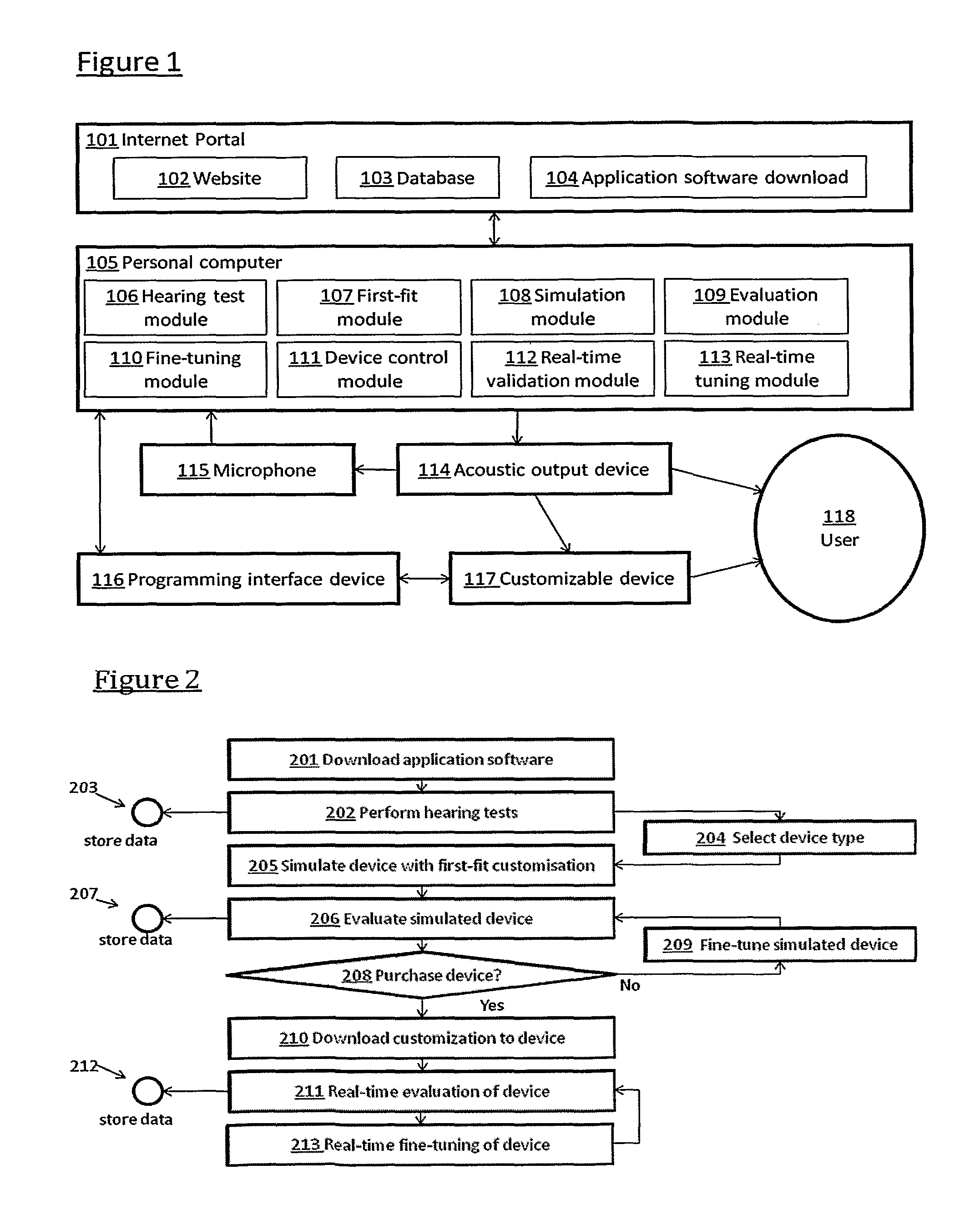
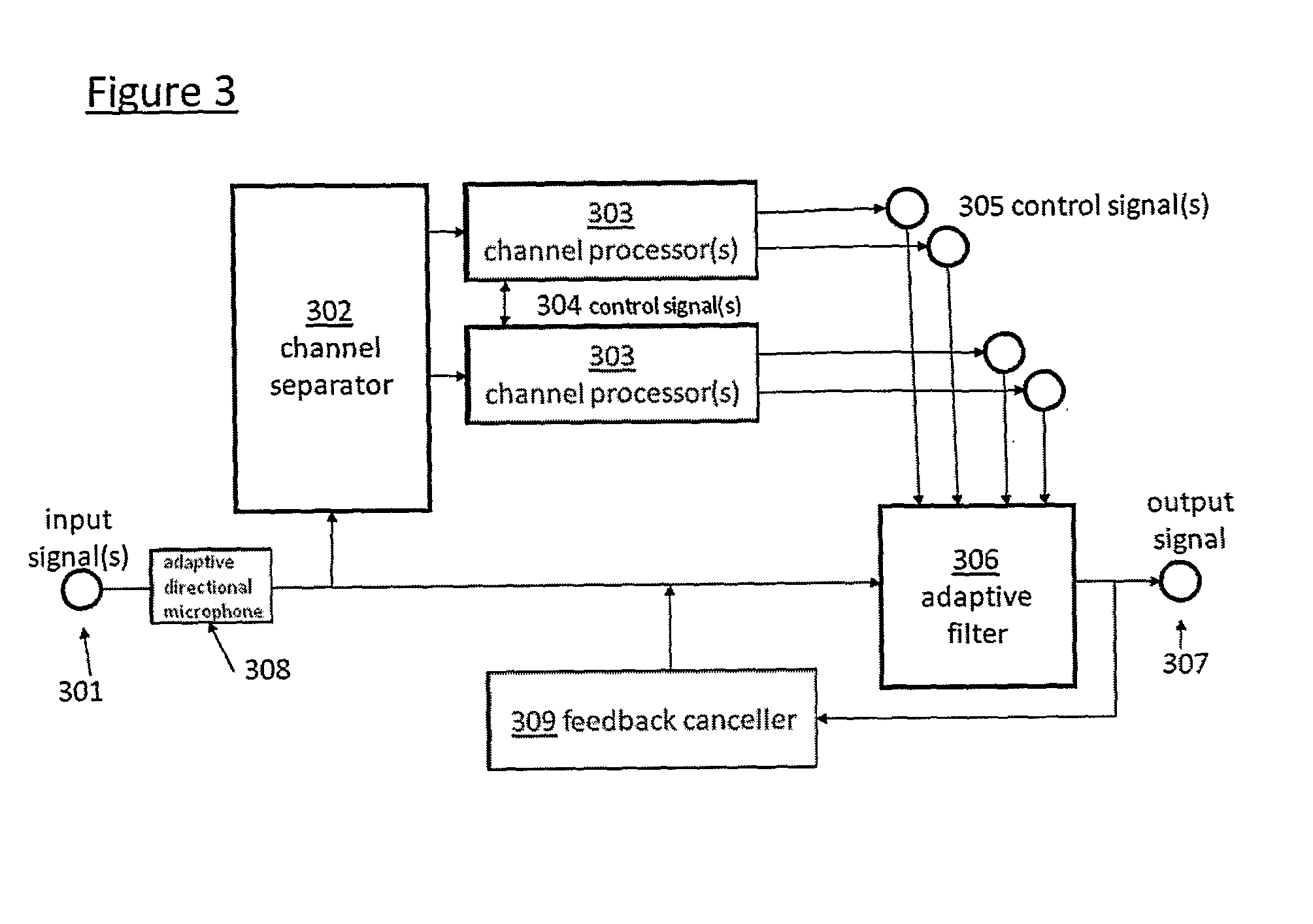
Automated fitting of hearing devices
ActiveUS20120051569A1Improve developmentEasy to adjustDeaf aid adaptationUser inputPattern perception
Fitting a sound processing device for an individual is automated using a computer. Fitting and customisation is carried out using natural sounds without specialised audiometric equipment or audiological expertise. Software for this purpose is downloaded from an internet portal. The computer plays back acoustic signals, and obtains user input reflecting the user's perceptions of the acoustic signals, from which a hearing map is derived, representing the user's hearing. An algorithm updates the device fitting based on the hearing map. Also provided is pre-sale virtual device fitting, whereby a virtual signal processing path is established in the computer, reflecting a signal processing function of a sound processing device of interest to the user. An algorithm updates parameters of the virtual processing path, based on the hearing map. Audio signals passed through the virtual processing path are played back to the user, giving the user an acoustic indication of future device performance.
Owner:SONOVA AG
Television system having internet web browsing capability
InactiveUS7590998B2Two-way working systemsSelective content distributionTelevision systemWeb browser
A television system for providing standard television programming and Internet browsing includes a television set for viewing digital and / or analog programming and includes a memory storage device and an integral embedded web browser for displaying data received from an Internet portal. The data is in the form of URLs and web pages that are synchronized with selected TV programming and / or advertising. A remote control selectively displays URLs and / or their associated web pages stored in memory by the Internet portal. In one aspect of the invention, TV programming can be displayed simultaneously on the screen with related web pages and URLs.
Owner:SHARP KK
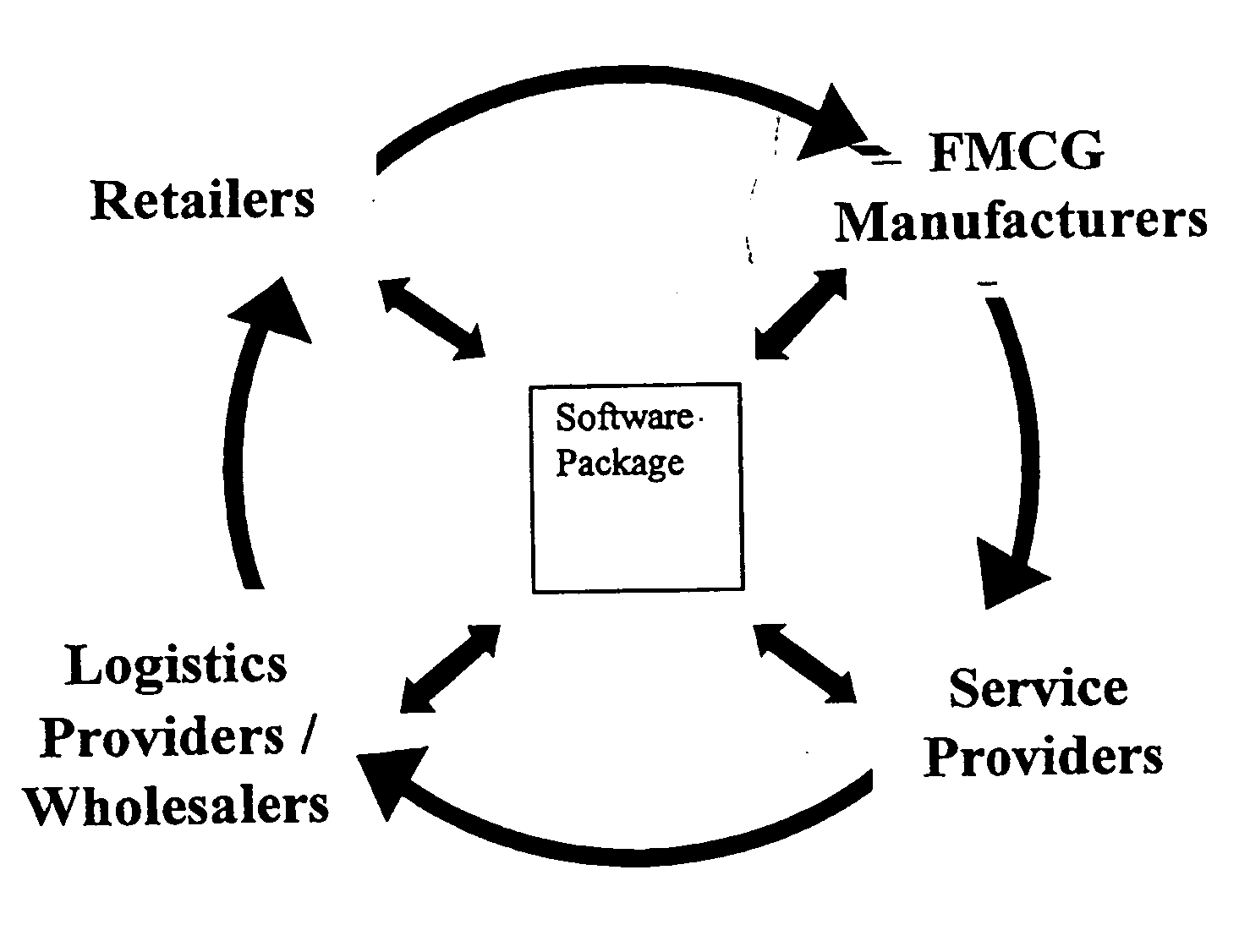
Network based business to business portal for the retail convenience marketplace
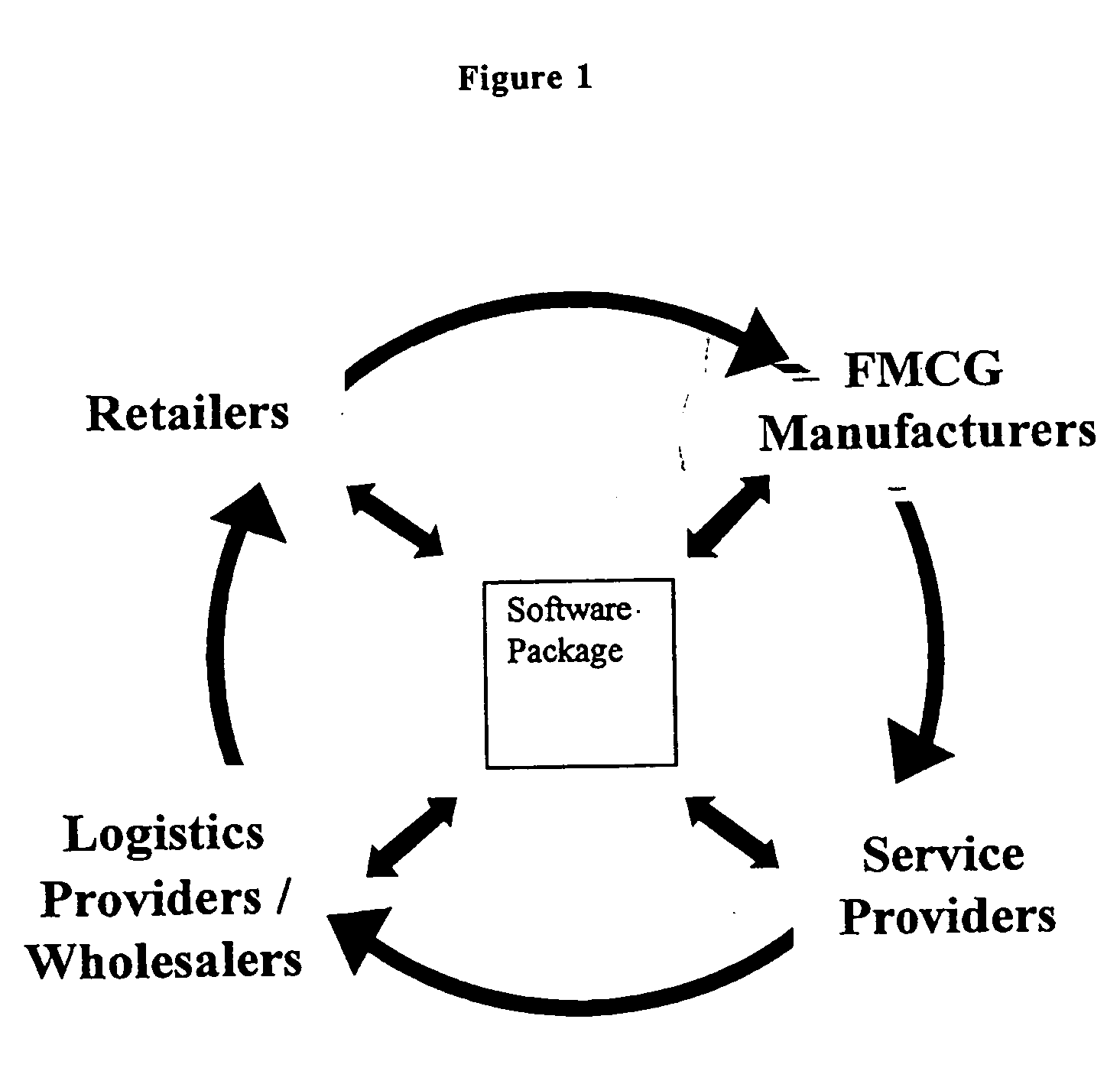
Broadly, the invention provides an Electronic Marketplace Solution (EMS) which may be embodied, in part or in whole, as a method, system or computer readable medium of instructions. The invention provides a business to business Internet portal serving companies operating within the convenience marketplace. In one embodiment, the invention is an Internet portal which assists: Independent Convenience Retailers through the provision of a single system to sell, buy and pay for goods / services and manage their business more effectively; Organised Convenience Groups, by providing them access to improved network management, the ability to ensure compliance and the ability to reduce costs; FMCG Manufacturers in the areas of secondary supply chain efficiencies, by improved business intelligence and direct access to Convenience Retailers; Wholesalers / Logistics Providers, for reduced costs and improved efficiency and enabling them to expand their customer base; and / or Service Providers, by enabling them to more efficiently reach a large target market.
Owner:BRITISH AMERICAN TOBACCO AUSTRALASIA
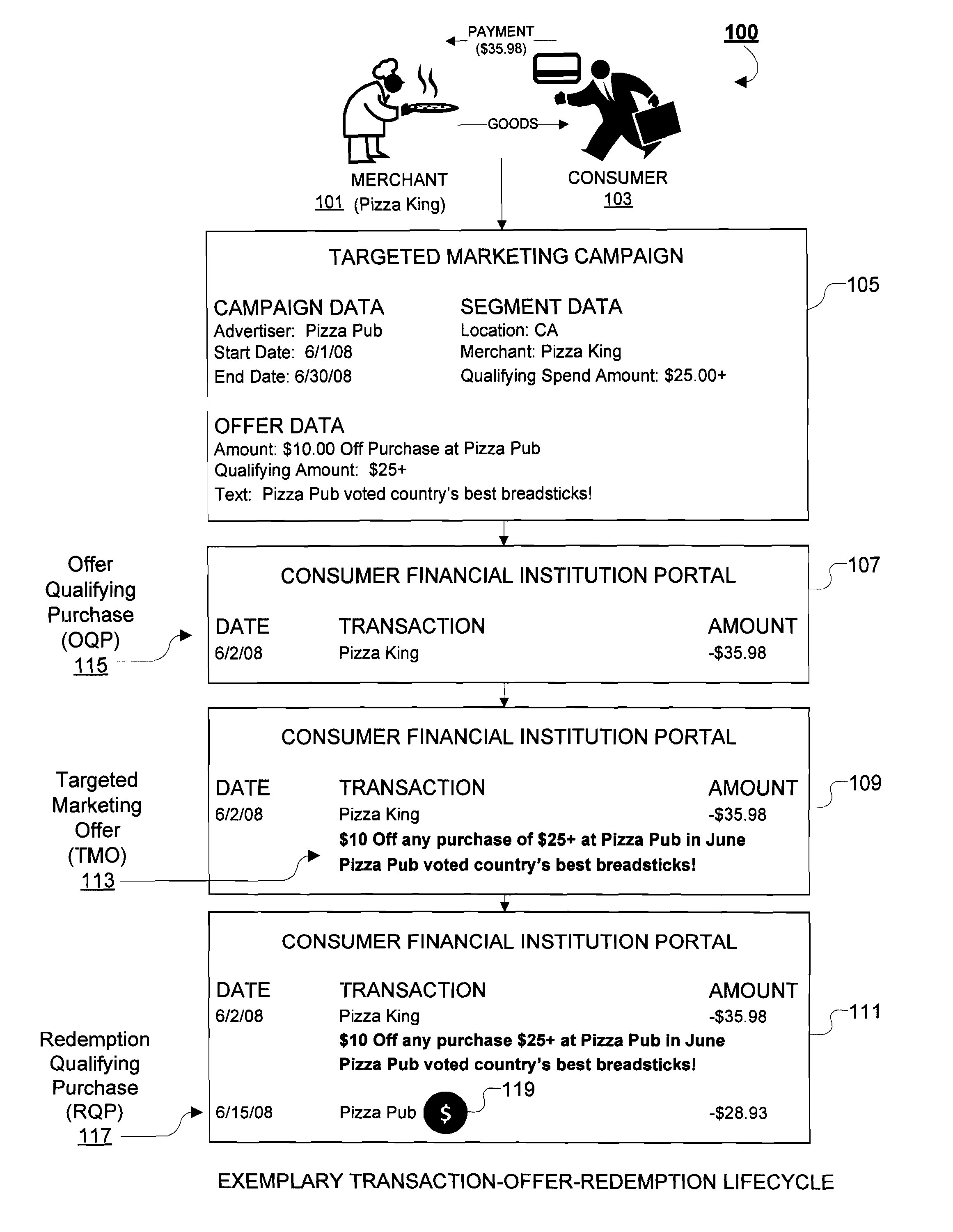
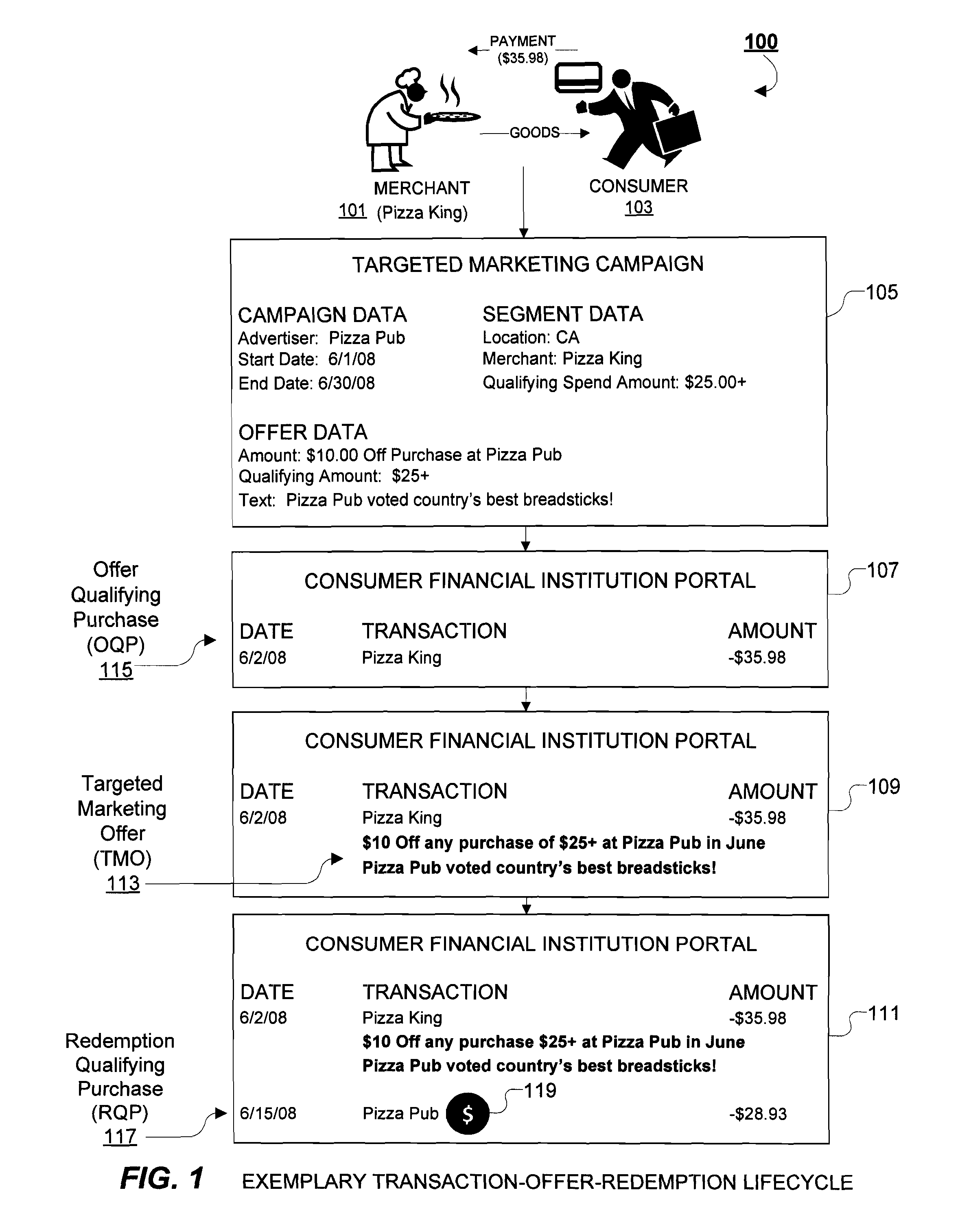
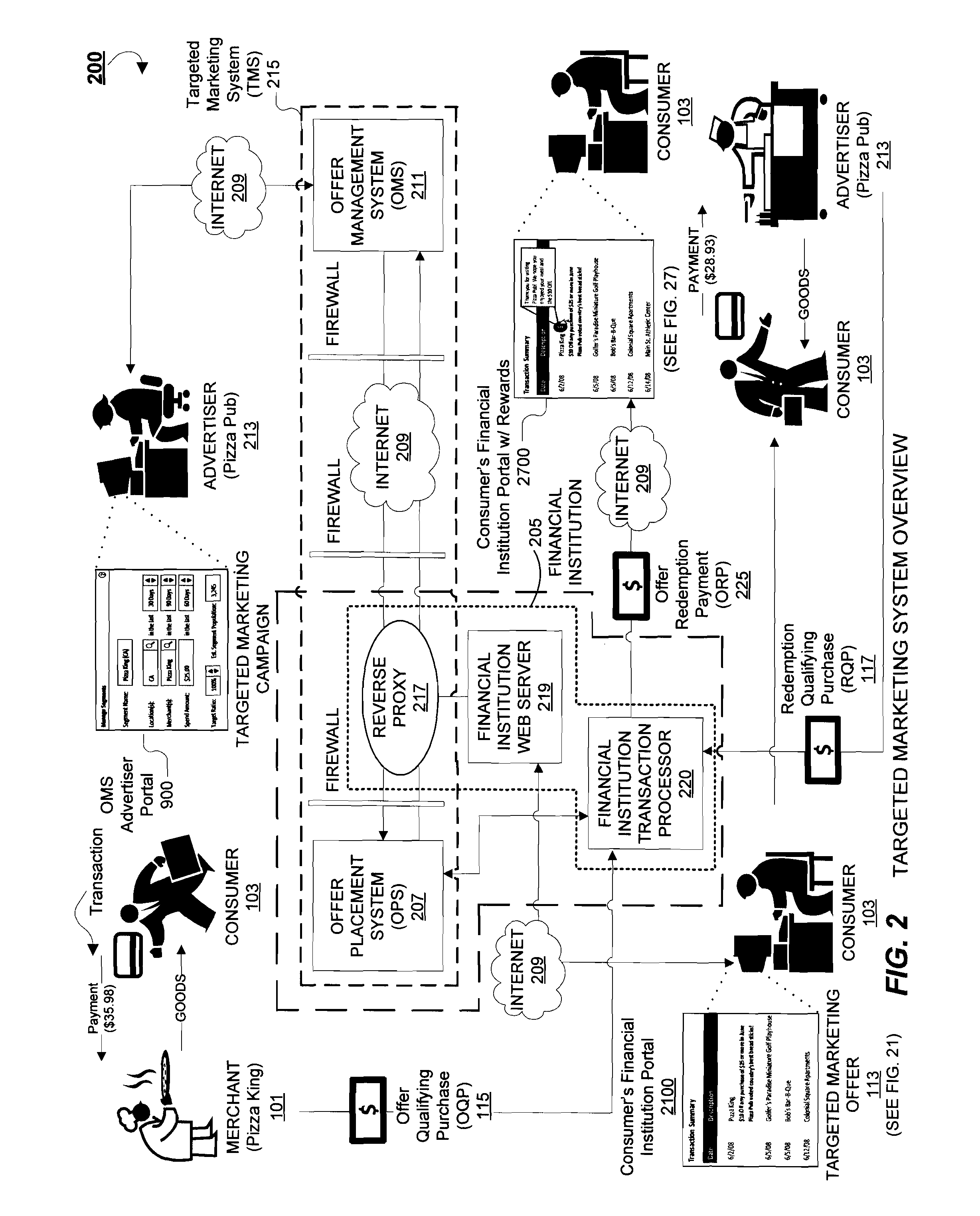
System and Methods for Delivering Targeted Marketing Offers to Consumers Via an Online Portal
A system and methods for delivering targeted marketing offers to consumers during a session with an online (web-based) Internet portal, particularly suitable for online banking portals of financial institutions. An offer management system receives information corresponding to an advertising campaign of an advertiser corresponding to terms of a targeted marketing offer to be provided to a consumer accessing the online portal, and provides advertising campaign data corresponding to the targeted marketing offer and to an offer-triggering event to an offer placement system. An offer placement system receives the advertising campaign data, determines the occurrence of the offer-triggering event by a consumer during an online session with the online portal, and delivers information corresponding to the targeted marketing offer to the consumer. In response to the offer-triggering event, such as display of a list of transactions, the predetermined targeted marketing offer is delivered to the consumer during the online session.
Owner:CARDLYTICS
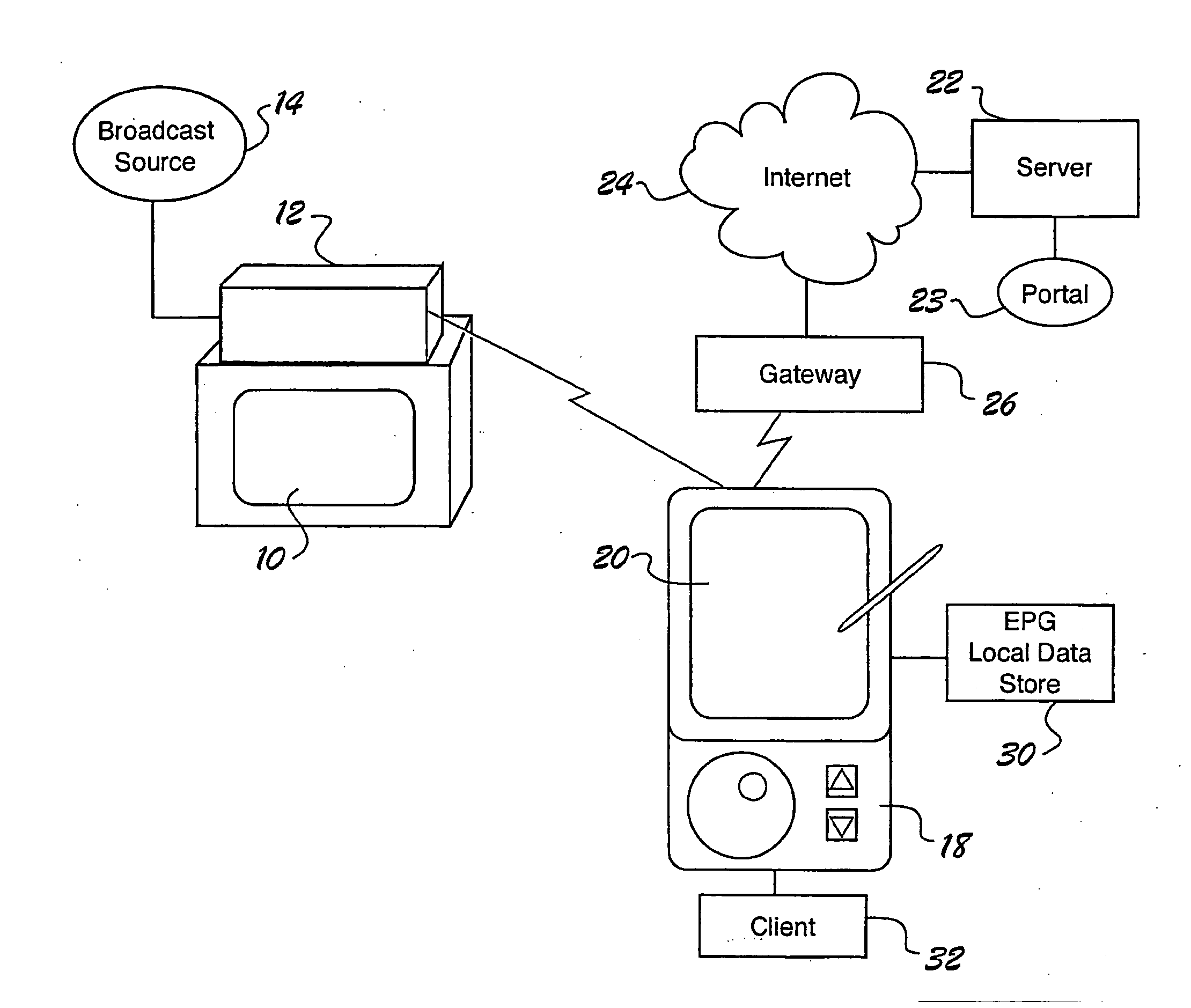
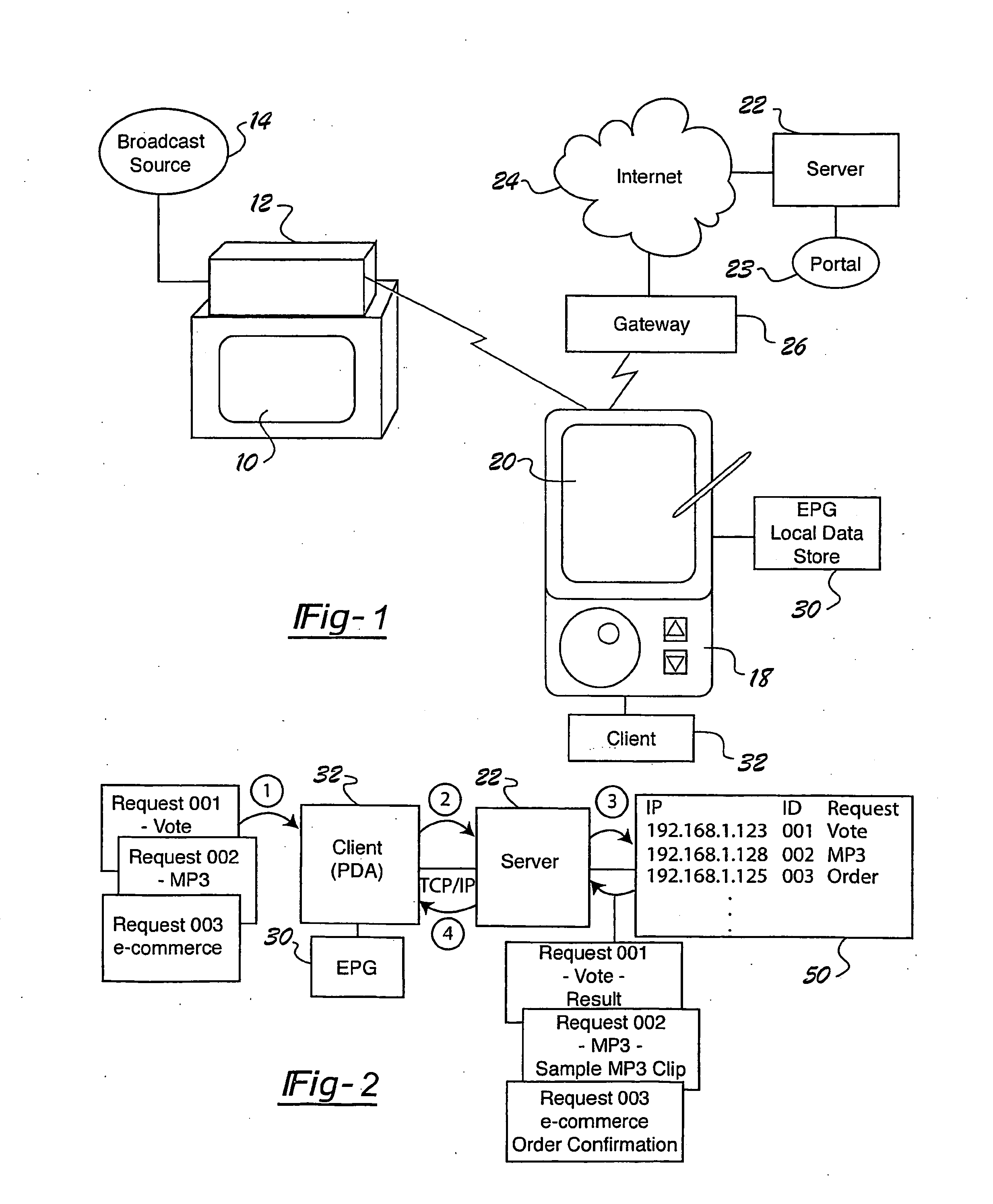
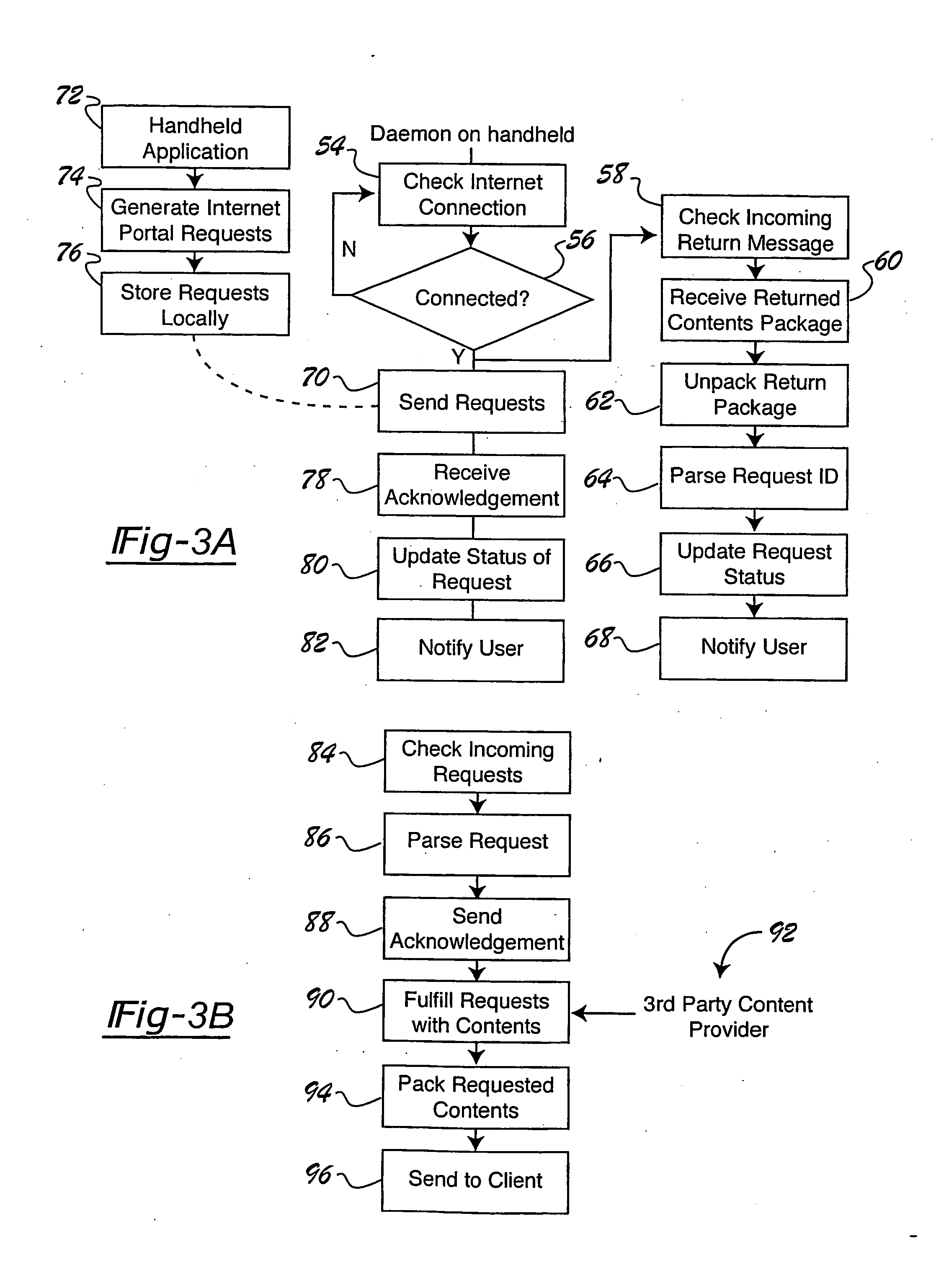
Internet portal system and method employing handheld device that connects to broadcast source
A portal system employs a handheld media delivery device (18) adapted to receive broadcast media content having media content information, adapted to formulate a request based on the media content information, adapted to communicate the request for additional media content to the portal system (23), adapted to receive the additional media content from the portal system (23), and adapted to deliver the additional media content to a consumer (32). The handheld device has a request processor adapted to determine whether a connection to the portal system is available, queue requests locally, and store requests until the connection is available. The portal system includes an input adapted to receive a request for additional media content from the handheld media delivery device (18), and a retrieval mechanism adapted to retrieve additional media content based on the request. An output is adapted to communicate the additional media content to the handheld media delivery device (18), thereby supplementing the media content.
Owner:PANASONIC CORP
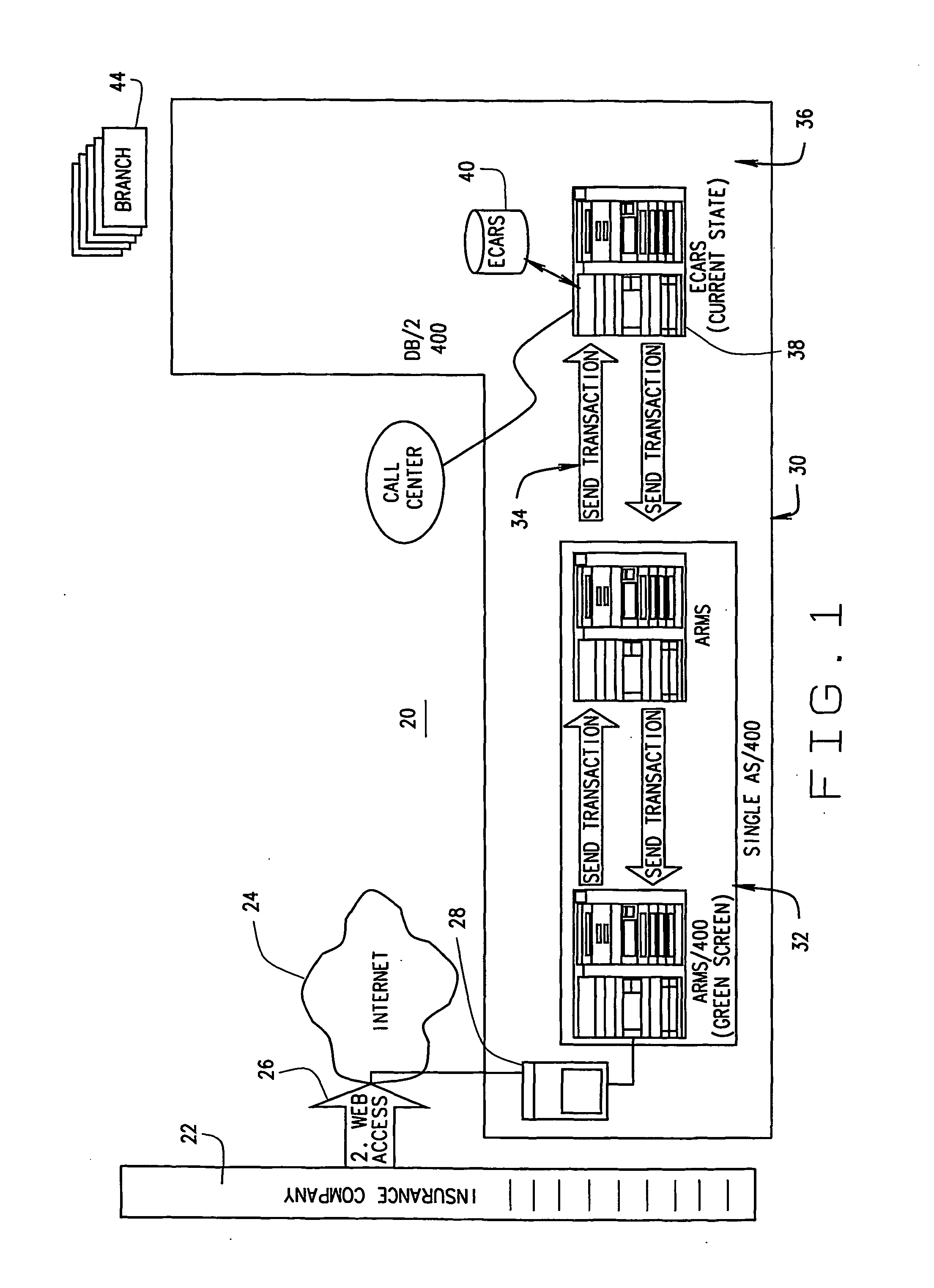
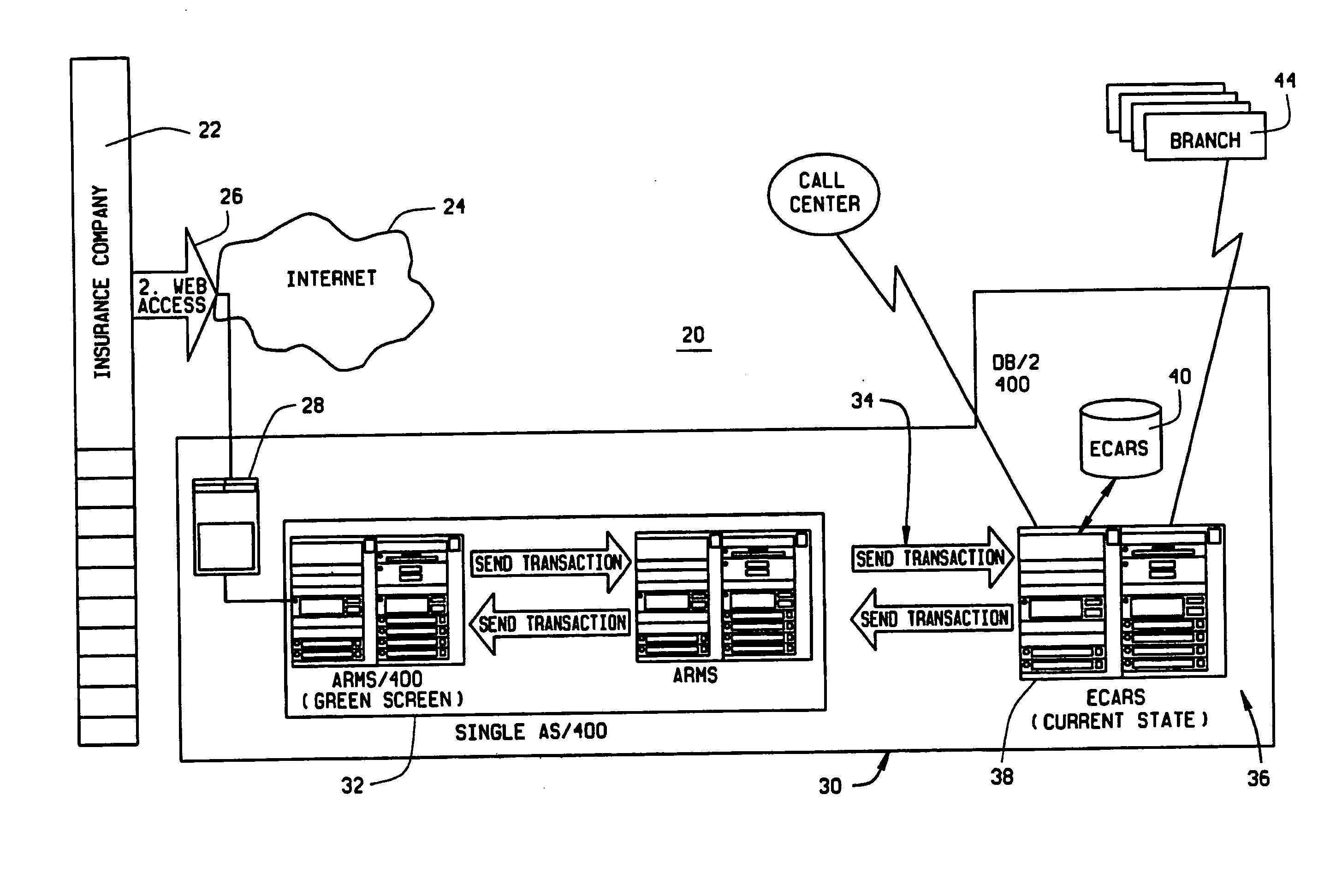
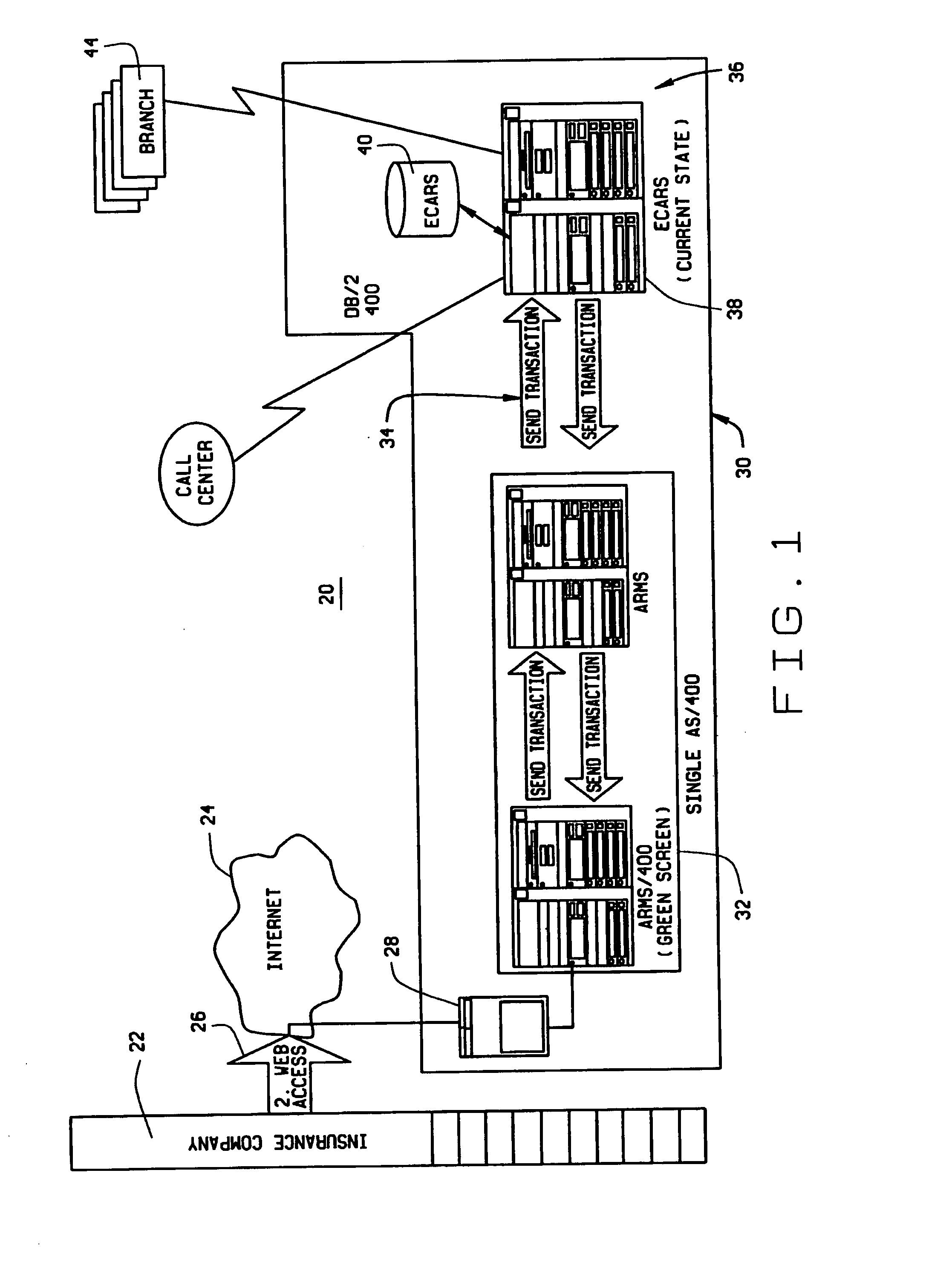
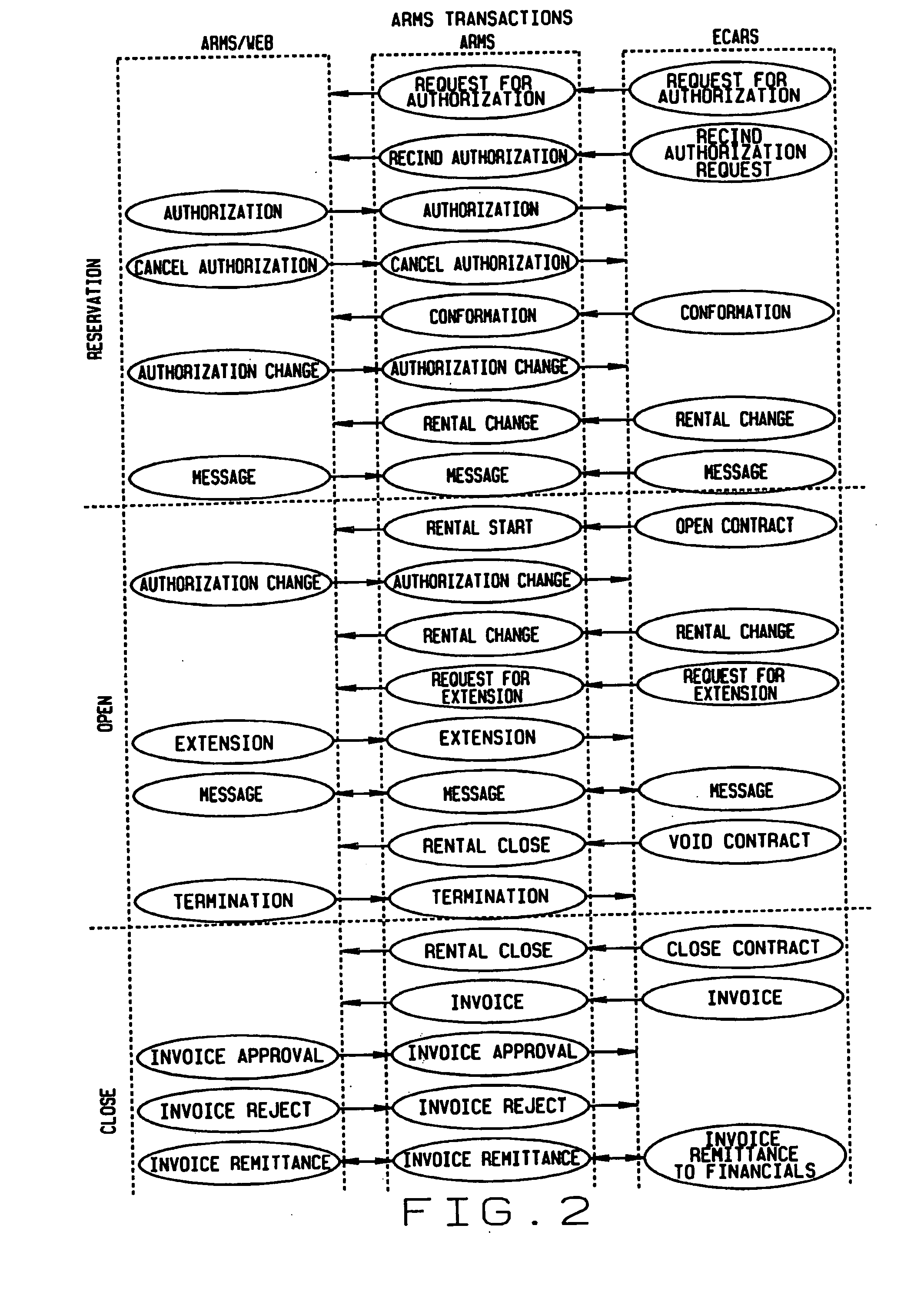
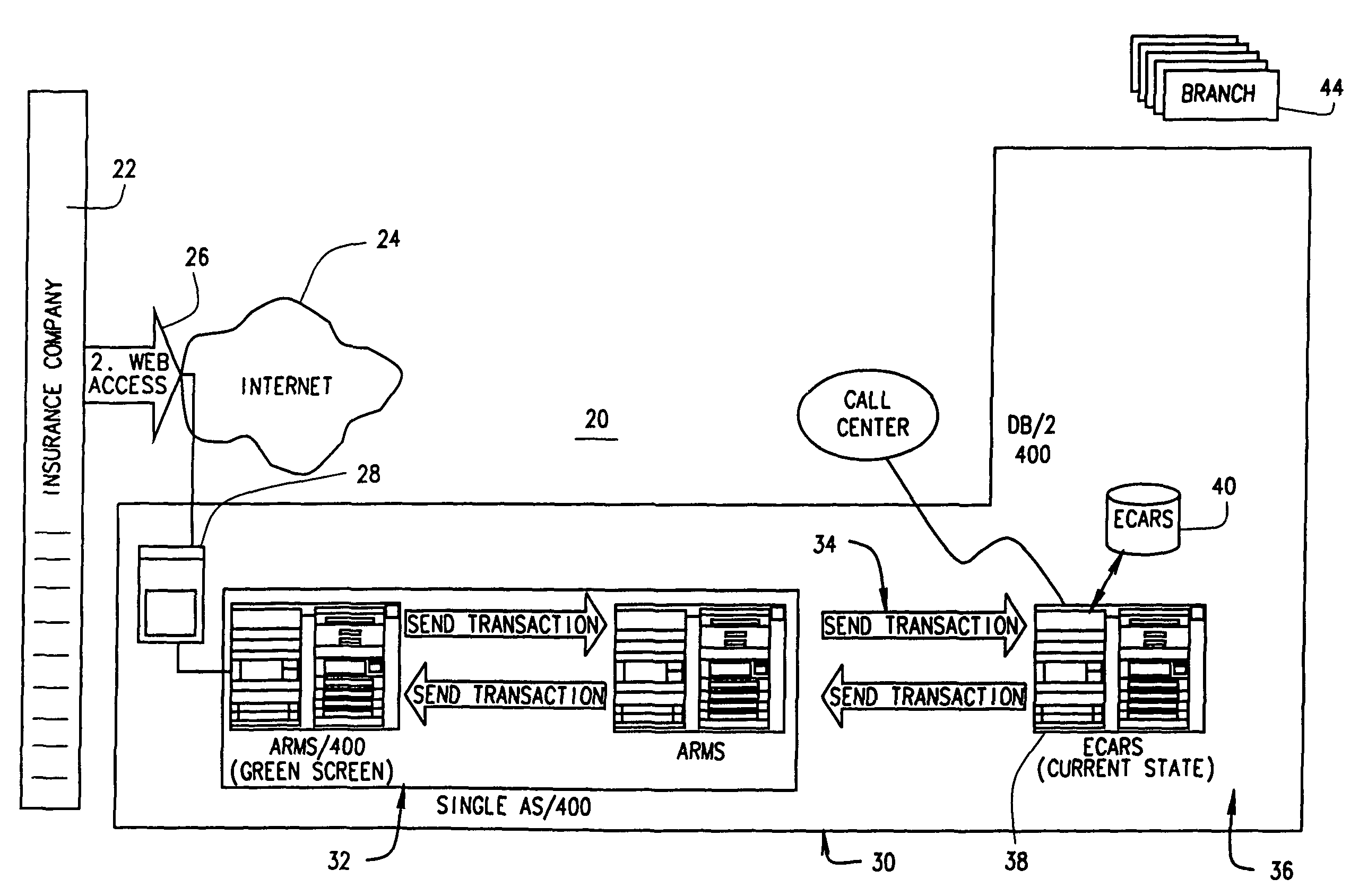
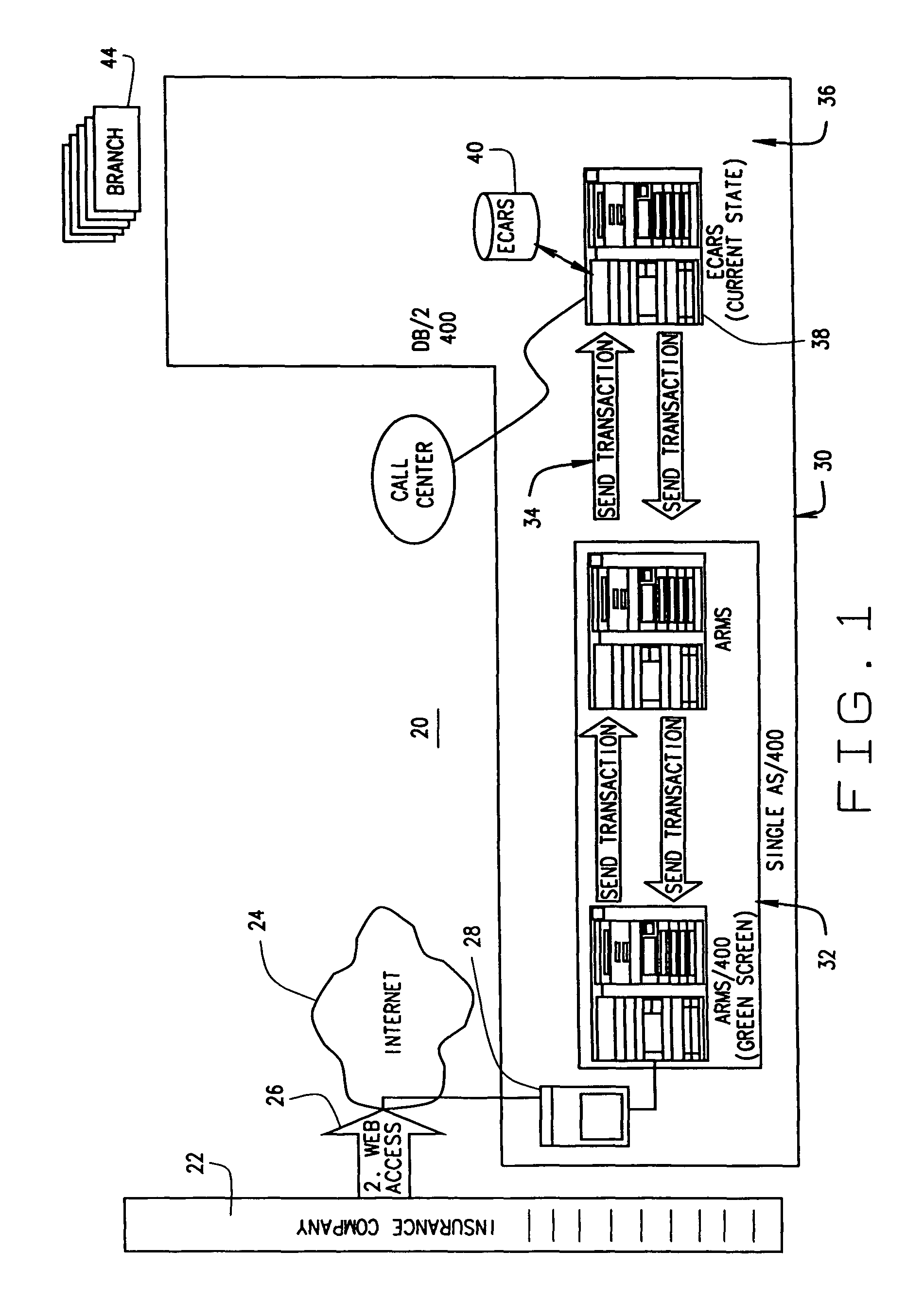
Extended web enabled multi-featured business to business computer system for rental vehicle services
ActiveUS20050021378A1Facilitate communicationExpense of was eliminatedFinanceReservationsPaymentTime schedule
An Internet enabled, business-to-business computerized transaction system is disclosed in its preferred embodiment for use in providing rental car services for high volume users and comprises an Internet web portal through which the high volume user may access a plurality of service providers including an integrated business computer network for at least one rental vehicle service provider. The rental vehicle services provider computer network is configured to interconnect a geographically diverse plurality of branch offices, cataloguing their available rental vehicles and schedules for same as well as handling all transactional data relating to its business. The Internet web portal provides ubiquitous connectivity and portability for a multi-level business organization who regularly places high volumes of rental purchases with its business partner and also those other service providers who may or may not have the same integrated business computer system and software. Utilizing the method and apparatus of the present invention large volumes of rental transactions may be placed, monitored, altered during performance, and closed out with financial accounting and payment being made virtually without human intervention.
Owner:THE CRAWFORD GROUP
New mobile communication data traffic charging mode
InactiveCN101741579AIncrease motivationFlexibleMetering/charging/biilling arrangementsData processing applicationsOperation modelApplication service provider
The invention provides a new mobile communication data traffic charging mode and relates to a user mobile terminal access mobile internet, various services on a communication network are used and charging modes of various contents are obtained, referring to the operation model of a content provider; the invention provides a charging mode that the flow fee of the data traffic in the communication network can be co-paid by mobile terminal users, mobile internet portals, application service provider, media, entertainment company and other content providers (CP / SP) or is independently paid by the content providers; the invention can improve the data traffic using activity of the terminal users, promote the increasing of the mobile data traffic, improve the popularizing rate of the mobile internet and accelerate the arrival of the mobile internet and the mobile IP times.
Owner:李占胜 +1
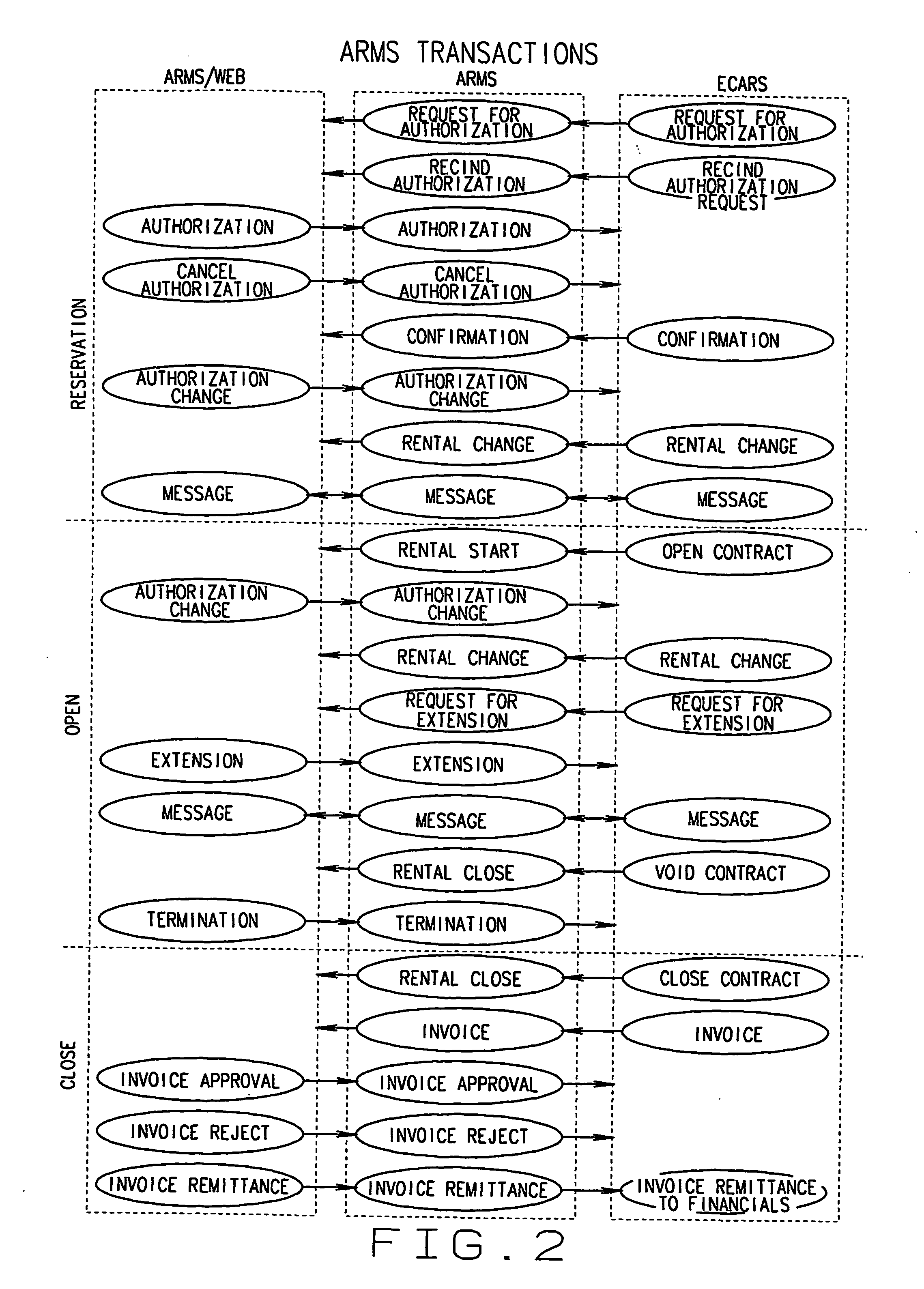
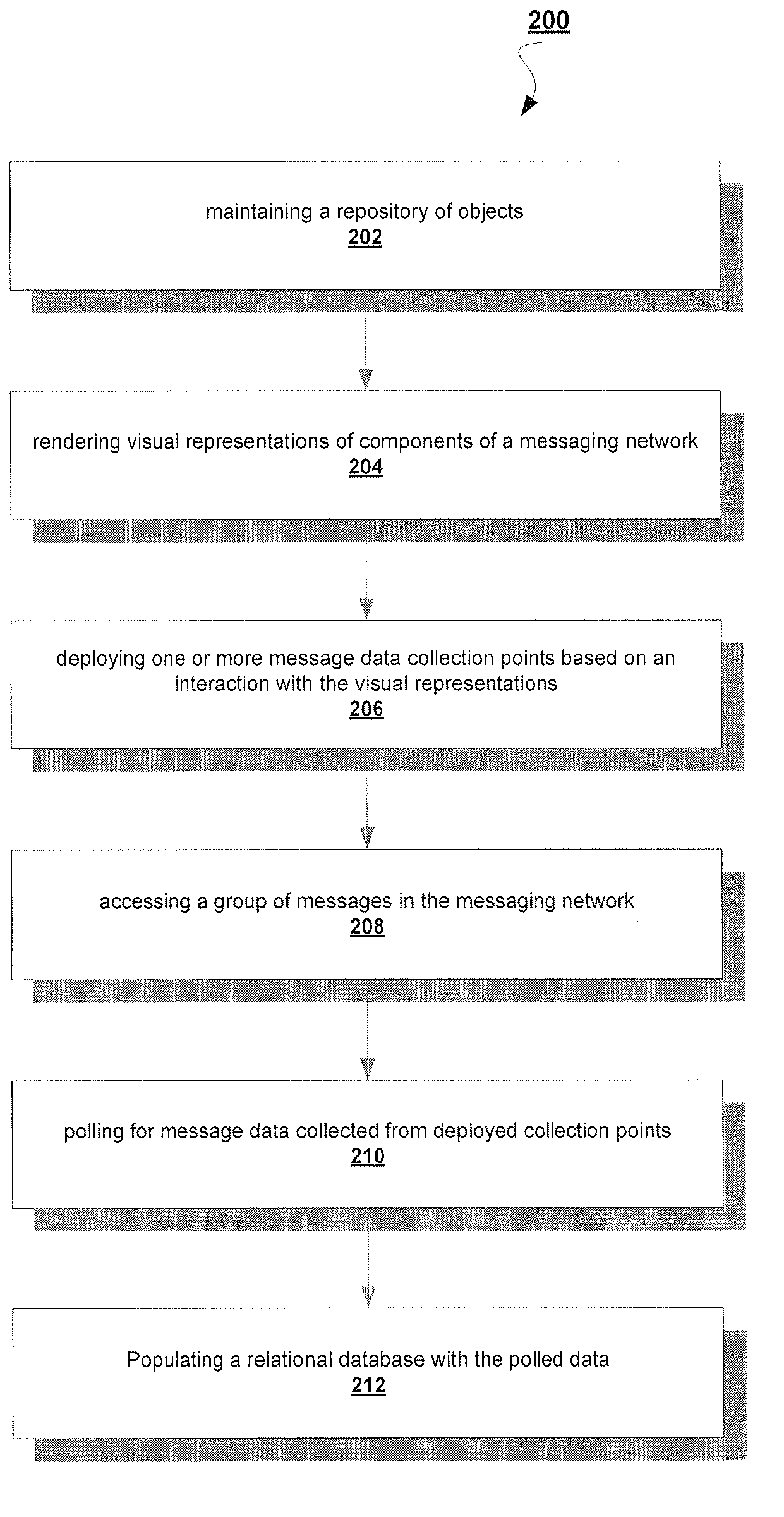
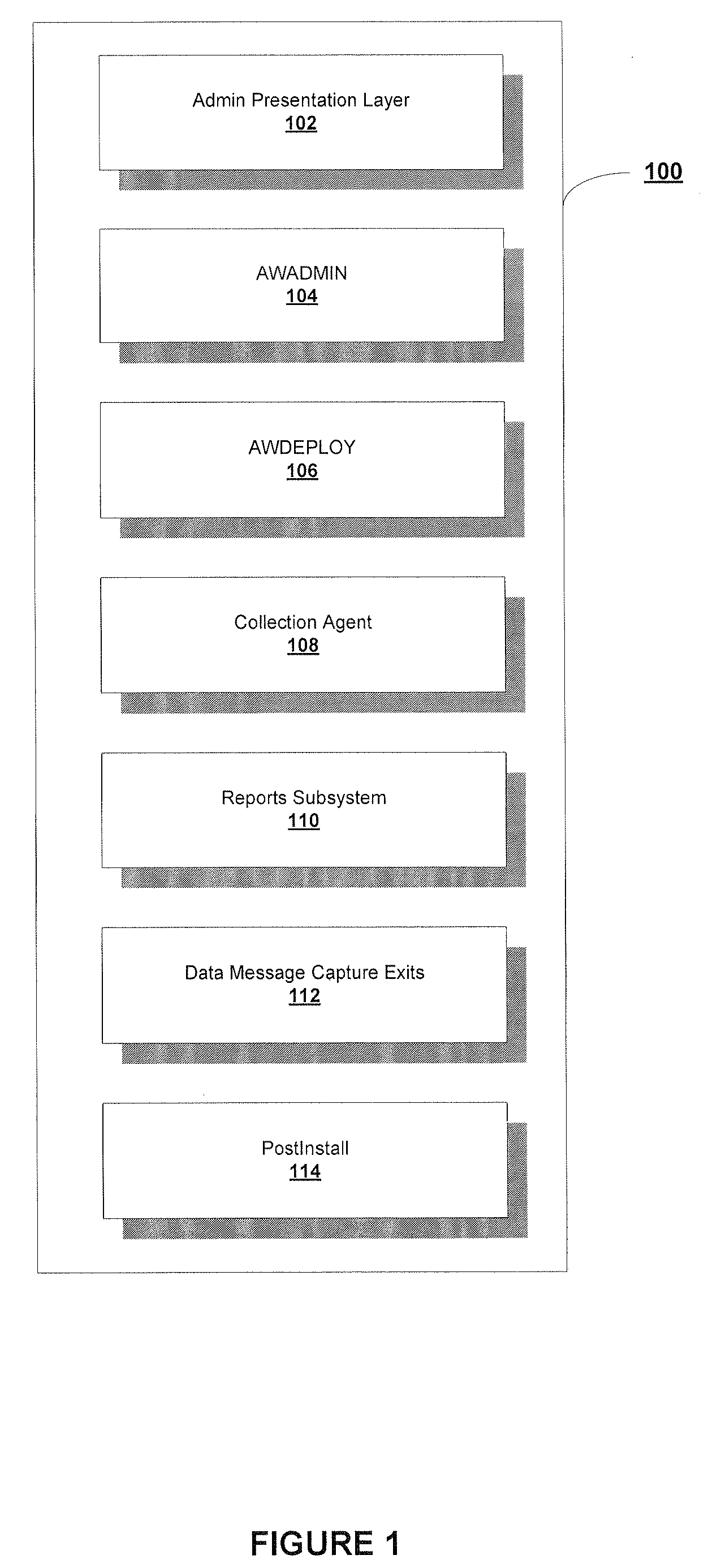
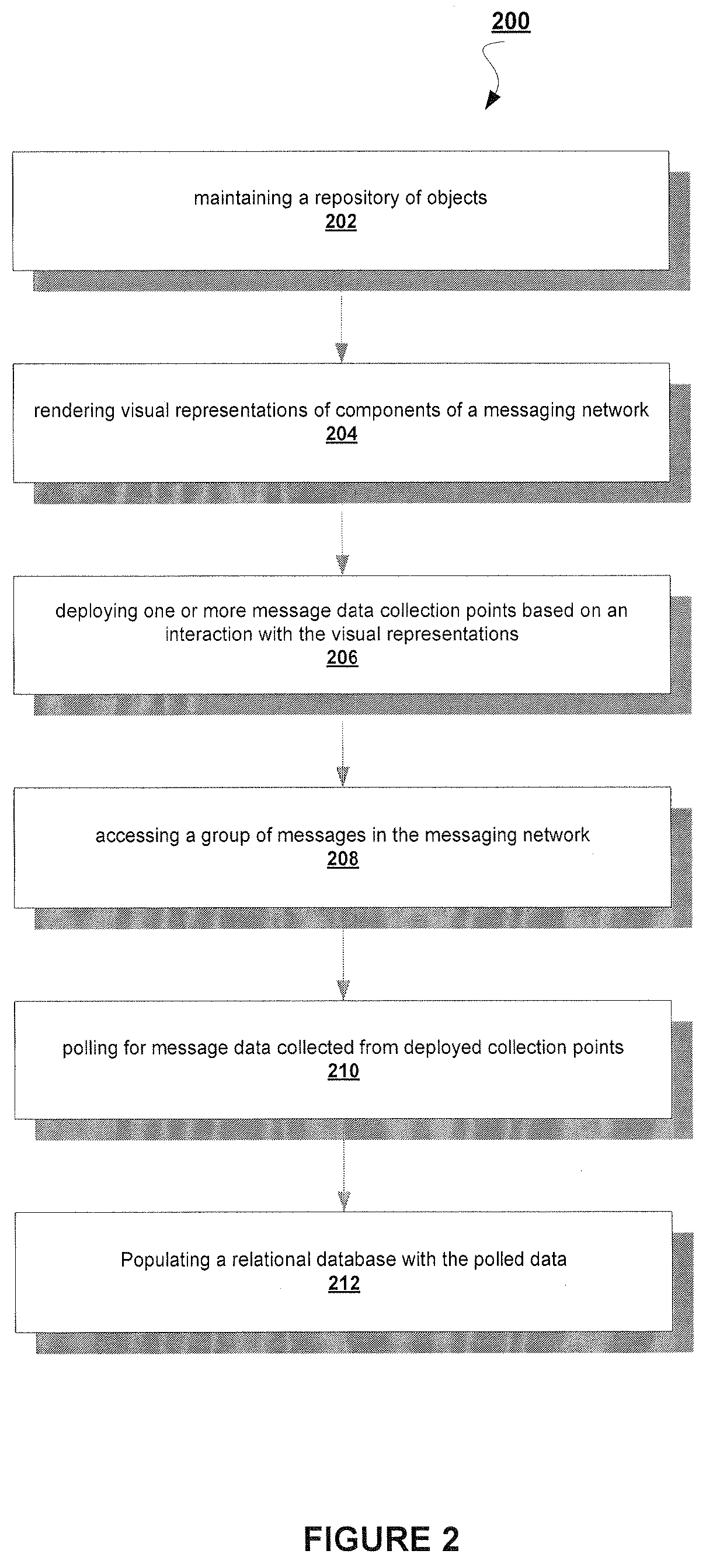
A System and Method to Capture, Filter, and Statistically Analyze Electronic Messages
InactiveUS20060047752A1Reduce overheadUseful operationComputer security arrangementsMultiple digital computer combinationsPrivate networkStatistical analysis
A business transaction management product which captures “snapshots” of messages and their payload content from a messaging network, on the fly, applies filters to select and abstract only priority messages / transactions, transfers the information over a private network or the Internet in a totally secure fashion and formats and stores the selected transaction data in a relational database, then provides performance analysis, usage analysis, detection of transactions misplaced in the infrastructure or delayed by errors. All performance analysis and transaction tracing information is provided through access to database reports for users via an Internet browser, similar to an Internet portal.
Owner:RECONDA INT CORP
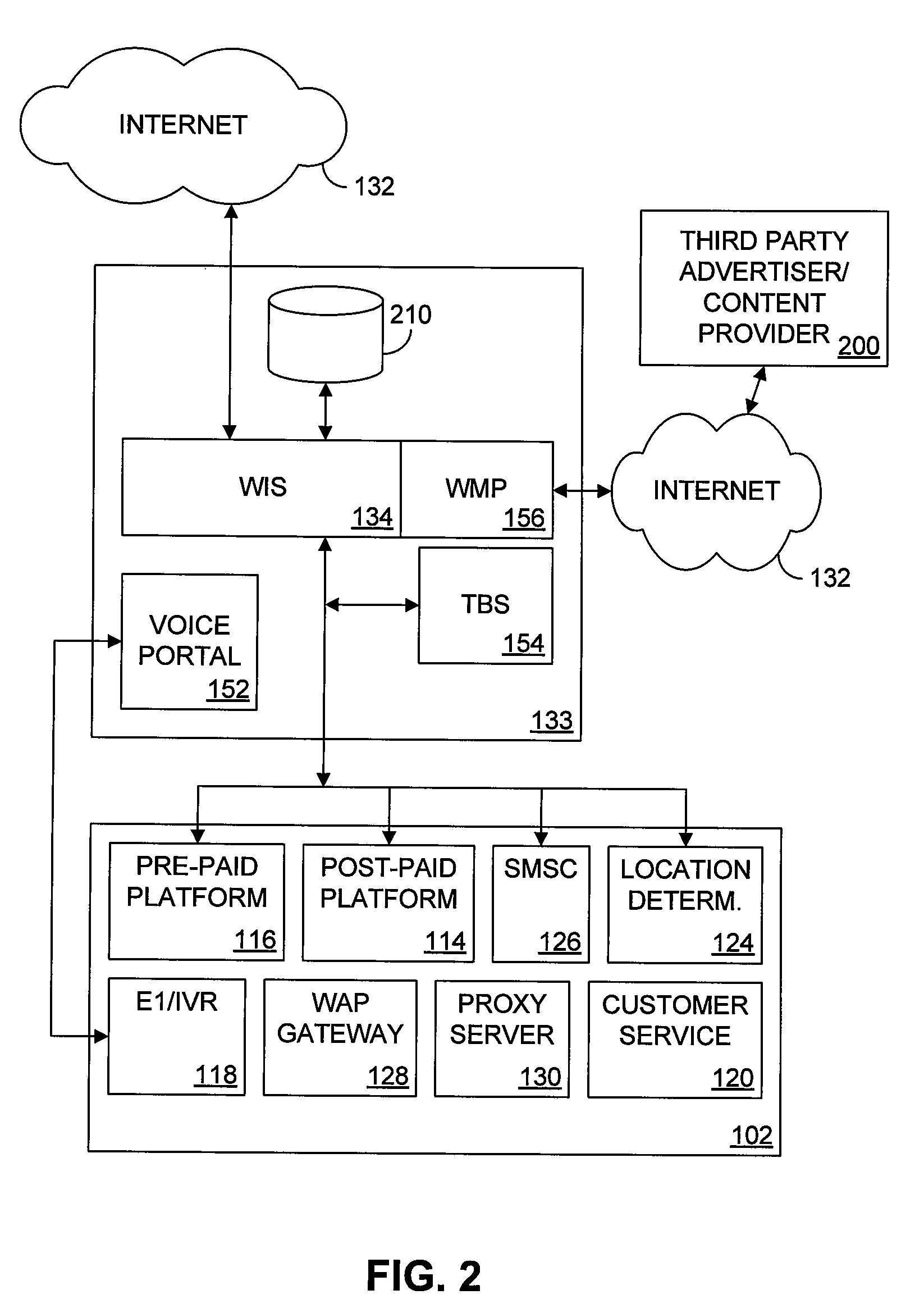
Seamless Multiple Access Internet Portal
InactiveUS20080229399A1Network traffic/resource managementDigital data processing detailsPersonalizationMobile Web
Multiple access internet portals are provided. A representative system, among others, includes a communication facility and a wireless internet server. The communication facility is operable to connect to a plurality of wireless devices through a mobile network. The wireless internet server is coupled to the communication facility and retrieves a personalized profile associated with a registered user an one of the plurality of wireless devices, and provides substantially similar personalized content to said at least one registered user on a variety of platforms associated with the wireless devices. Methods and other systems for multiple access portals are also provided.
Owner:AT&T DELAWARE INTPROP INC +1
Apparatus and method for creating communication group based on classification label
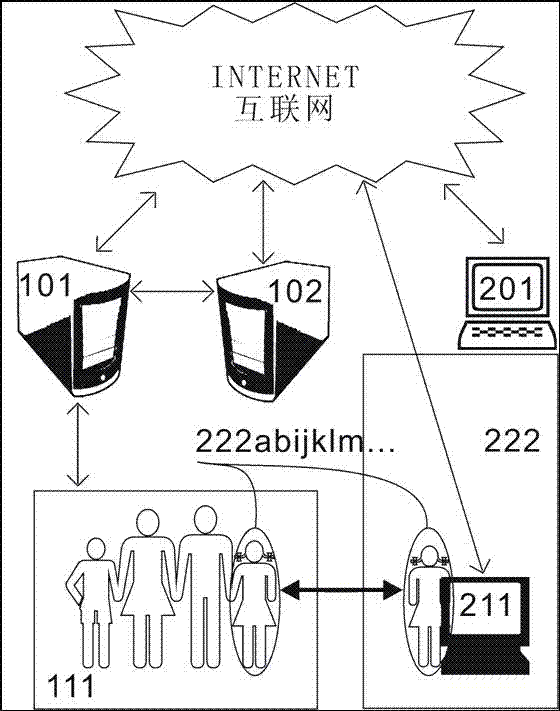
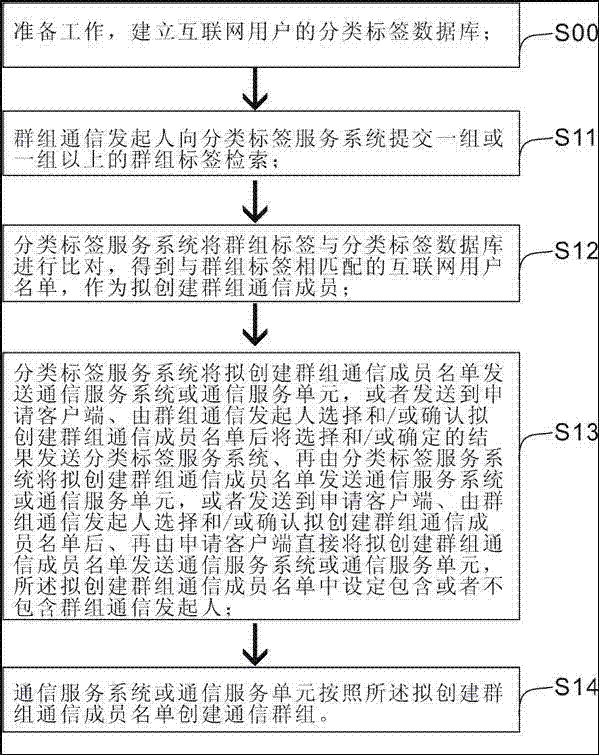
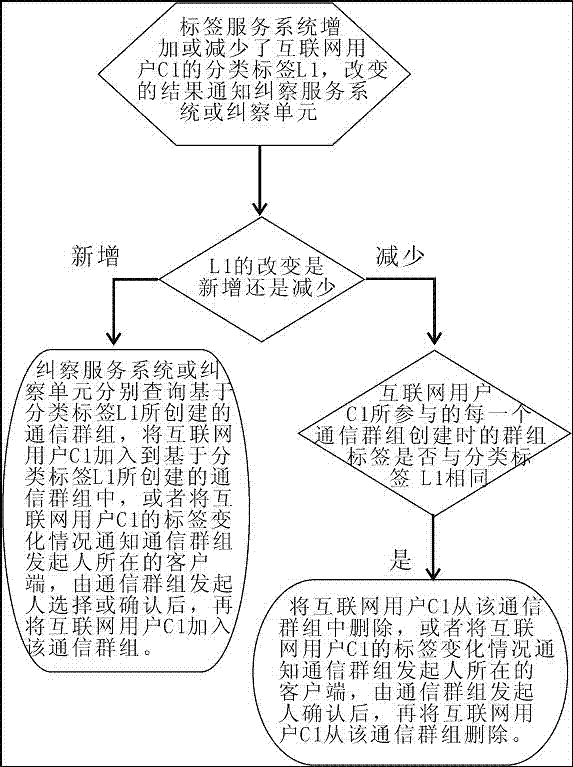
InactiveCN107317688ARealize group communicationTake the initiative to joinSpecial service provision for substationMetadata multimedia retrievalWeb pageInternet portal
The invention provides an apparatus and method for creating a communication group based on a classification label. Group label retrieval and classification label database greatly facilitate the creation of personalized groups, each search comprising browsers can realize group chat, and then realize online shopping, vehicle ordering and other Internet services through the group chat; the Internet services replace webpage search based on the group label search; and the group chat is converted into browsing and the Internet services, thus becoming a new Internet portal.
Owner:薛江炜
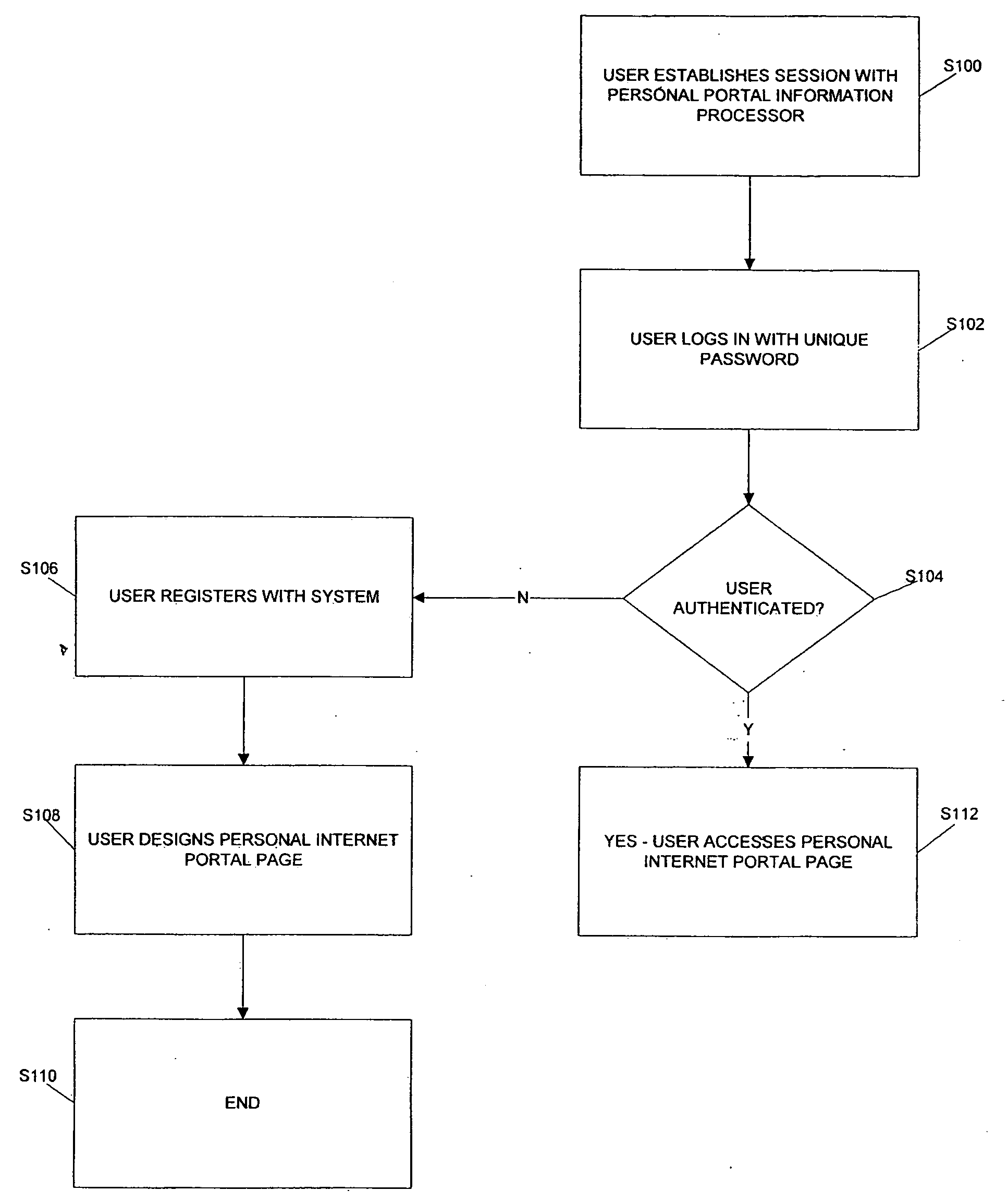
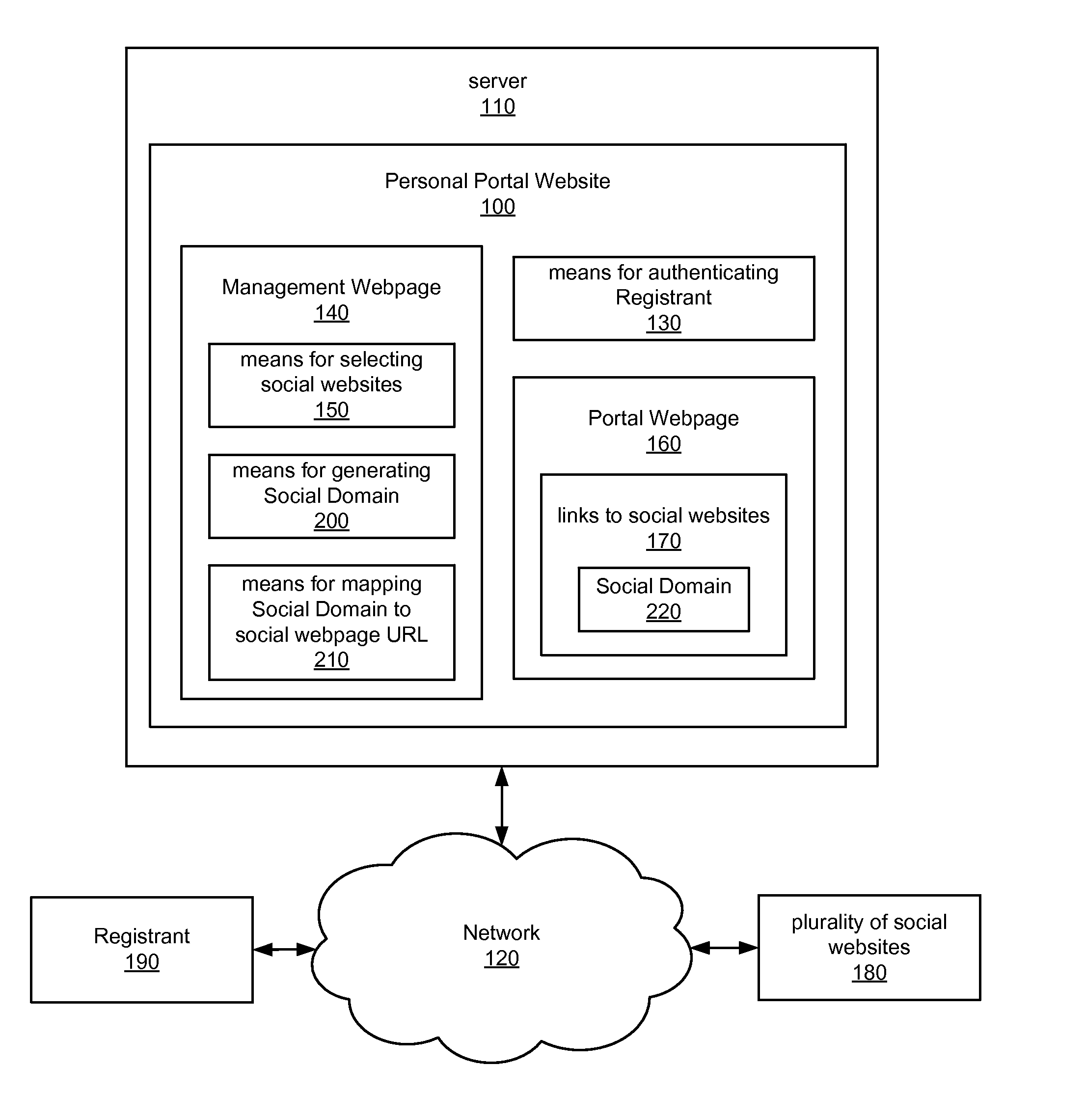
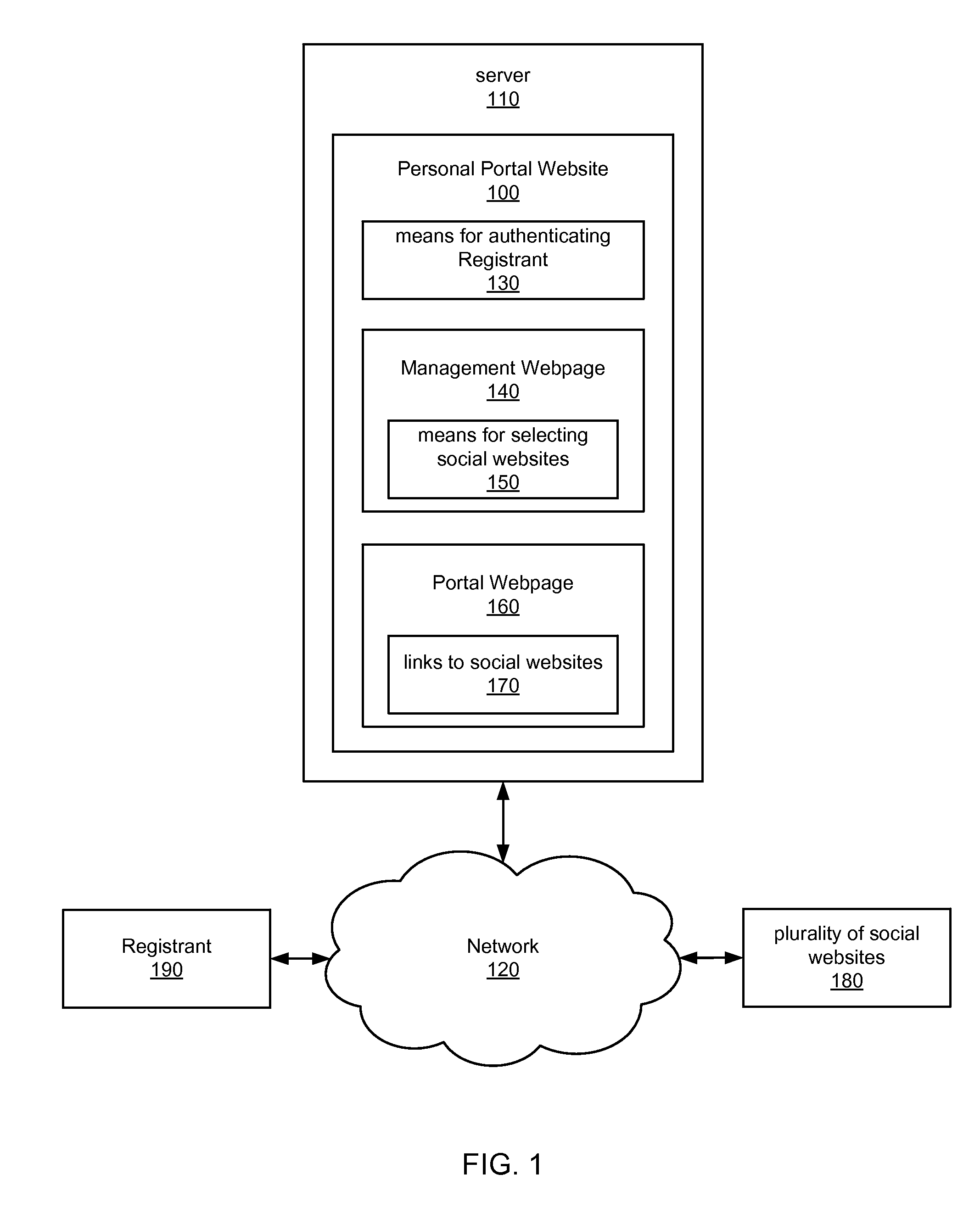
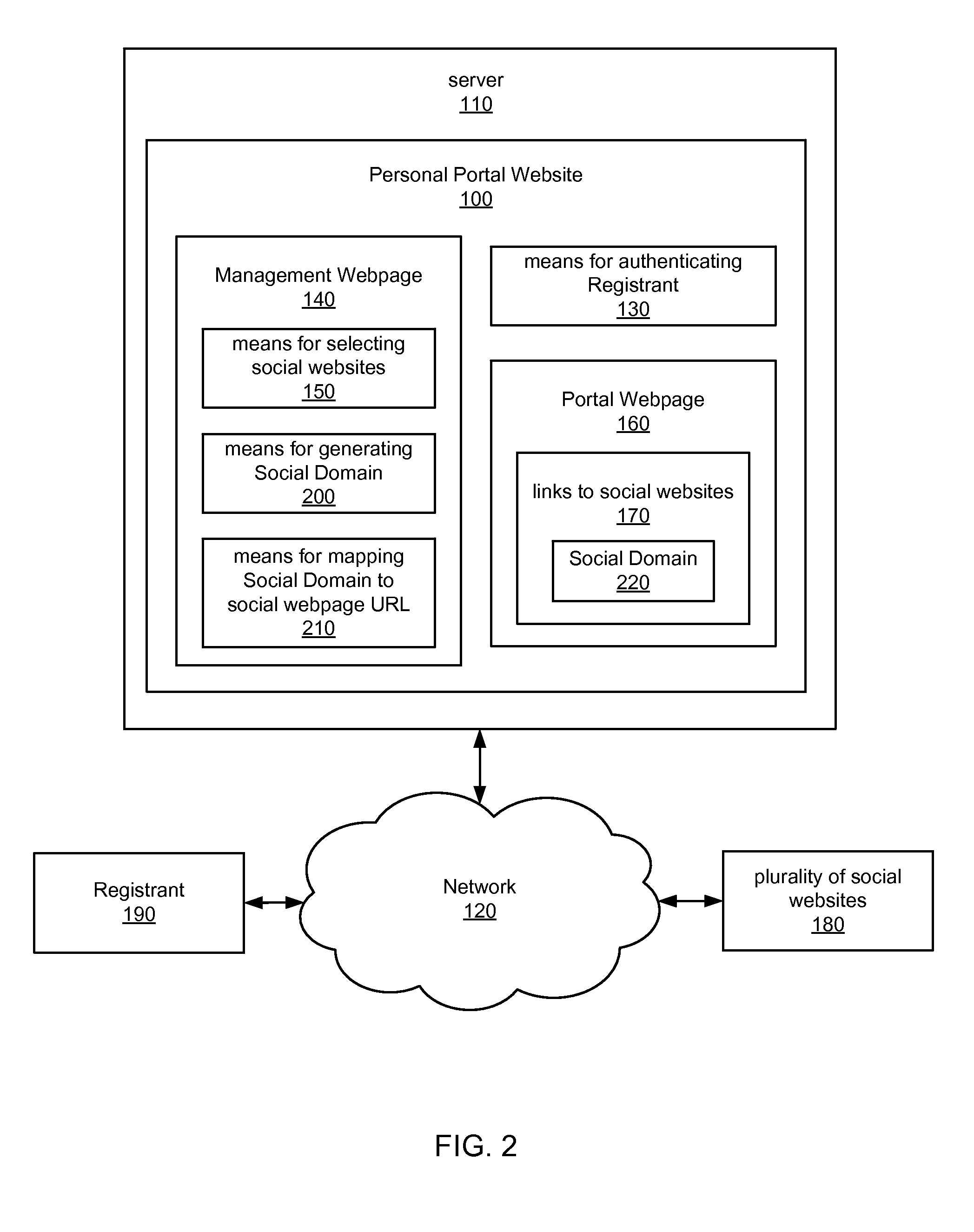
Internet portal for managing social websites
ActiveUS20080114867A1Efficient managementMultiple digital computer combinationsTransmissionDomain nameWeb site
Systems of the present invention allow for managing multiple social websites. An exemplary system may comprise a Personal Portal Website hosted on a server that may be communicatively coupled to a Network. The Personal Portal Website may resolve from a domain name registered to a Registrant. The Personal Portal Website also may have means for authenticating the Registrant and a Management Webpage, which is accessible only to the Registrant after successful authentication. The Management Webpage may also have means for selecting a plurality of social websites. A Portal Webpage also may be accessible via the Personal Portal Website, and may have links to the social websites selected by the Registrant on the Management Webpage.
Owner:GO DADDY OPERATING
Network-based bookmark management and WEB-summary system
InactiveUS20050210297A1Digital data information retrievalDigital data processing detailsClient-sideUniform resource locator
A network-based URL management and data gathering system is provided. The system utilizes a client-side utility for capturing URLs during normal Web browsing, and a server-side utility for organizing and managing the captured URLs on the network. The server-side utility periodically sends a request to a proxy browsing and data gathering utility for navigating to and retrieving data from Web pages associated with the captured URLs. Data retrieved from the Web pages is returned in summary form for presentation to subscribing users. In preferred embodiments, the system is practiced on the Internet network between users operating an Internet-capable appliance having an Internet connection, and an Internet portal service.
Owner:YODLEE COM INC
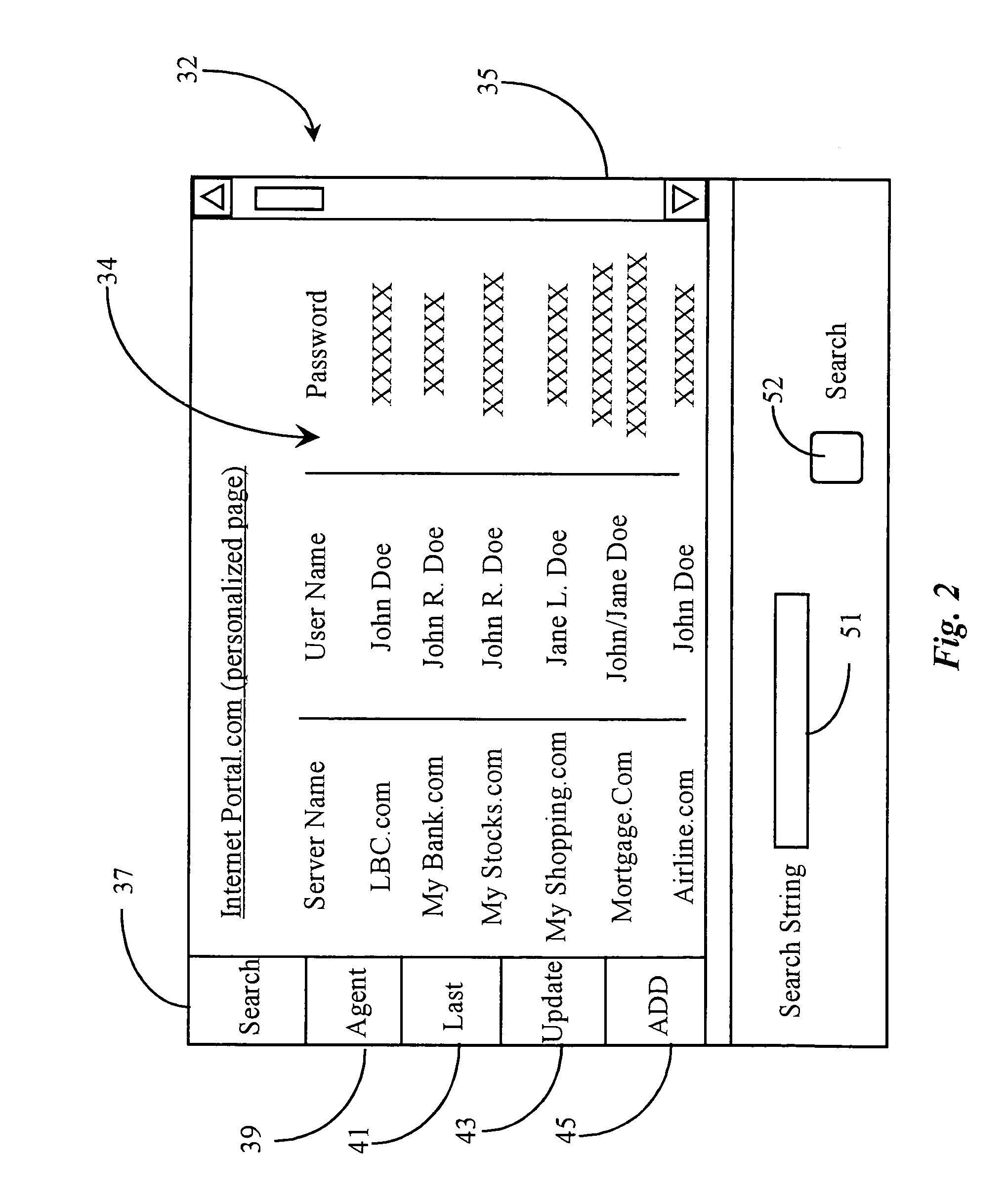
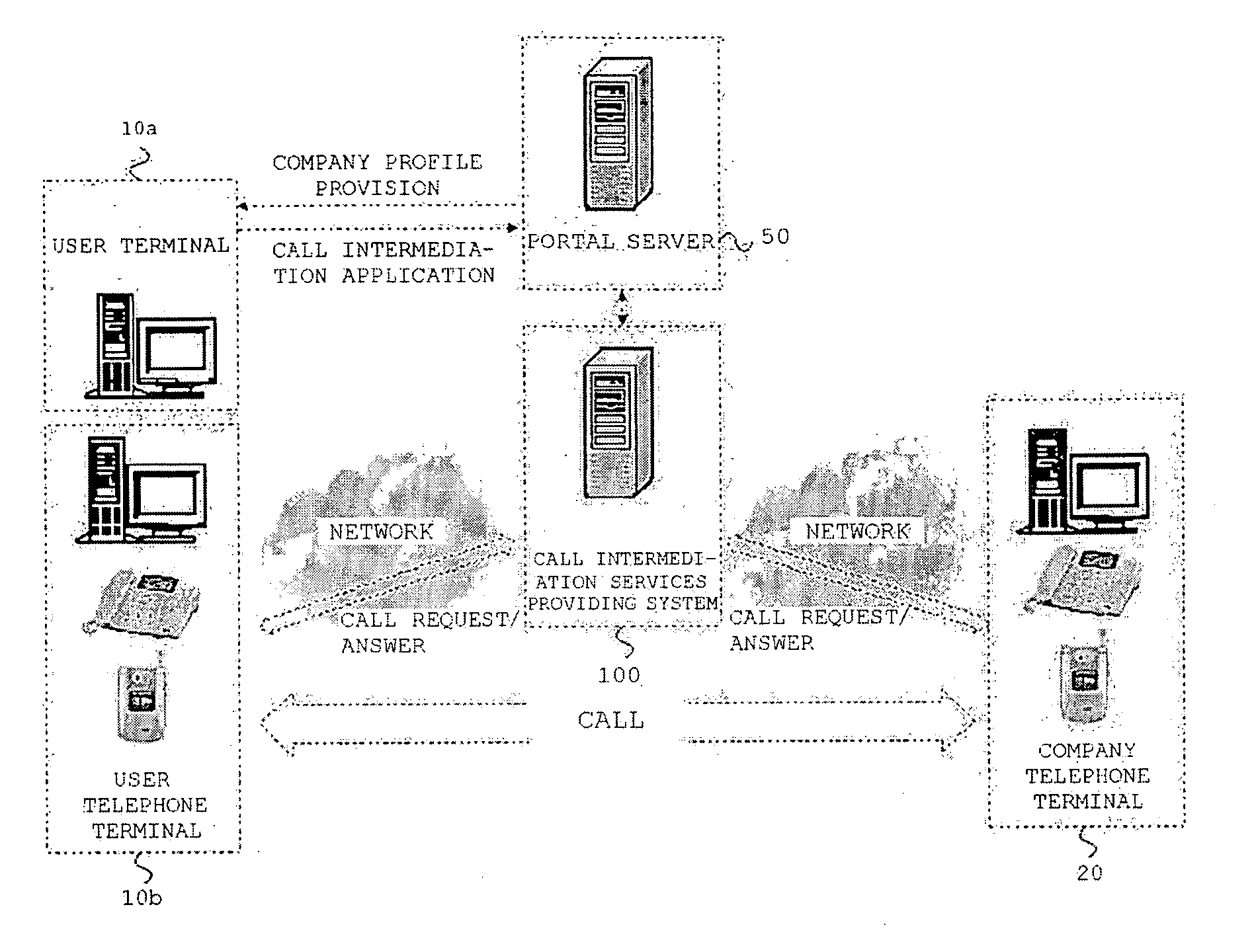
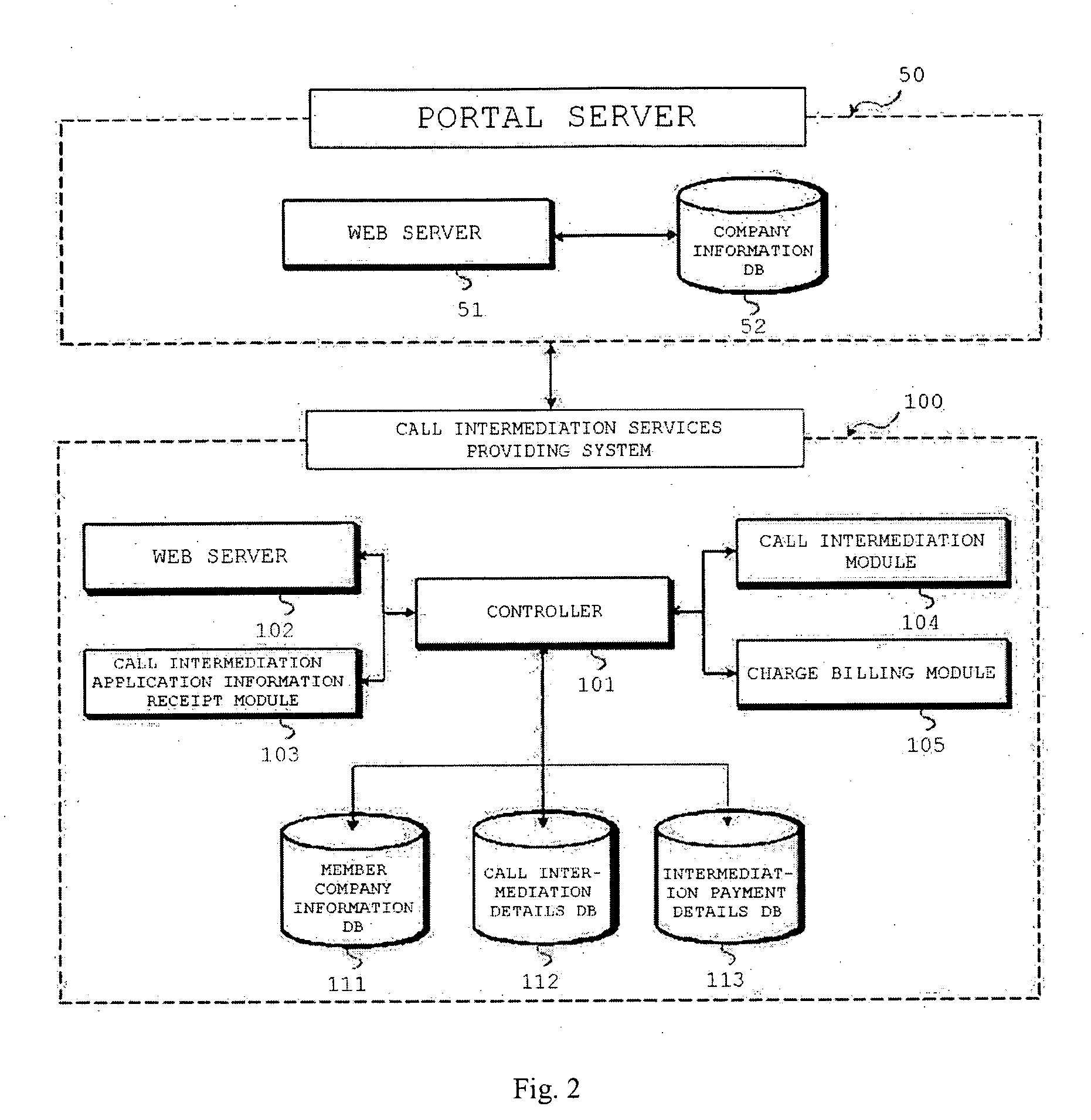
Method for providing call intermediation services and system therefore
Disclosed is a method and system for providing call intermediation services. The method for providing the call intermediation services allows a user to automatically make a call with a company if a user inputs only the telephone number on the web. After confirming the company profile of the Internet portal site, a user can speak by telephone with a person in charge of a company without a waiting time for call connection, and the user can rapidly acquire necessary information by means of query and response.
Owner:YUN
Multiple Access Internet Portal Revenue Sharing
InactiveUS20070042750A1Metering/charging/biilling arrangementsInterconnection arrangementsInternet accessMobile Web
Multiple access internet portal billing systems are provided. A representative system, among others, includes a communication facility, a wireless internet server, and a transaction billing system. The communication facility includes a billing platform, and is operable to connect to a plurality of wireless device platforms through a mobile network, and to connect to the wireless internet server. The wireless internet server provides internet access to the wireless devices and communicate at least one billing information record including a usage time to a transaction billing system. The transaction billing system receives the billing information record from the wireless internet server, formats the billing information record, and communicates the formatted record to the communication facility billing platform. Methods and other systems for multiple access internet portals are also provided.
Owner:AT&T INTPROP I LP
Web enabled business to business computer system for rental car services
An Internet enabled, business-to-business computerized transaction system is disclosed in its preferred embodiment for use in providing rental car services for high volume users and comprises an Internet web portal through which the high volume user may access an integrated business computer network for the rental vehicle service provider. The rental vehicle services provider computer network is configured to interconnect a geographically diverse plurality of branch offices, cataloguing their available rental vehicles and schedules for same as well as handling all transactional data relating to its business. The Internet web portal provides ubiquitous connectivity and portability for a multi-level business organization who regularly places high volumes of rental purchases with its business partner. Utilizing the method and apparatus of the present invention large volumes of rental transactions may be placed, monitored, altered during performance, and closed out with financial accounting and payment being made virtually without human intervention.
Owner:THE CRAWFORD GROUP
Extended web enabled business to business computer system for rental vehicle services
ActiveUS7899690B1Facilitate communicationExpense of was eliminatedInstruments for road network navigationFinanceBusiness-to-businessEngineering
An Internet enabled, business-to-business computerized transaction system is disclosed in its preferred embodiment for use in providing rental car services for high volume users and comprises an Internet web portal through which the high volume user may access a plurality of service providers including an integrated business computer network for at least one rental vehicle service provider. The rental vehicle services provider computer network is configured to interconnect a geographically diverse plurality of branch offices, cataloguing their available rental vehicles and schedules for same as well as handling all transactional data relating to its business. The Internet web portal provides ubiquitous connectivity and portability for a multi-level business organization who regularly places high volumes of rental purchases with its business partner and also those other service providers who may or may not have the same integrated business computer system and software. Utilizing the method and apparatus of the present invention large volumes of rental transactions may be placed, monitored, altered during performance, and closed out with financial accounting and payment being made virtually without human intervention.
Owner:THE CRAWFORD GROUP
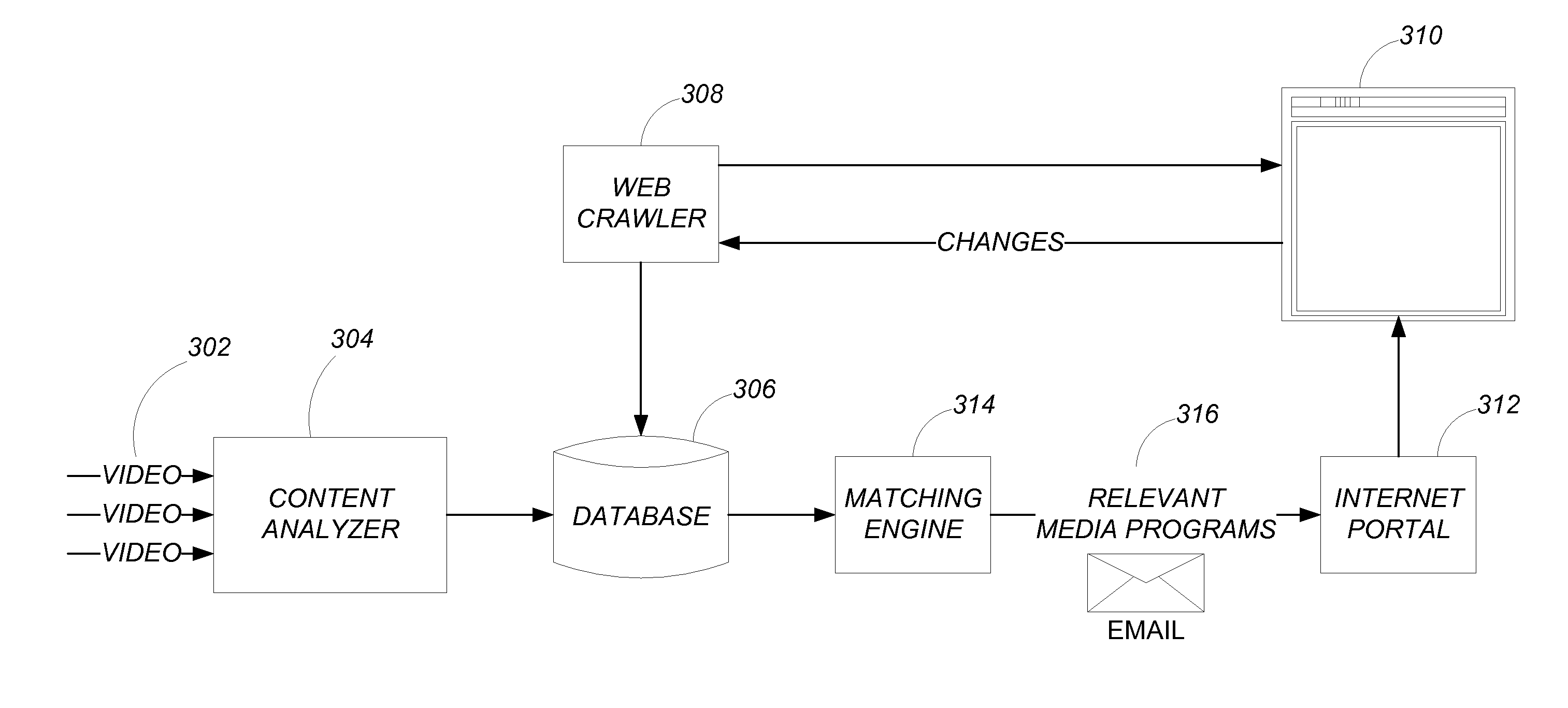
Semantic matching by content analysis
ActiveUS20120191692A1Digital data processing detailsWebsite content managementSemantic matchingContent analytics
A method, apparatus, system, article of manufacture, and computer readable storage medium provide media content. A web page context for a web page is determined and stored in a database. One or more media content files are analyzed to extract information that is stored in the database. The information is compared to the web page context. A matching media content file is determined from the one of the one or more media content files that matches the web page context based on the comparison. The matching media content file is then provided (e.g., to an internet portal web site).
Owner:HULU
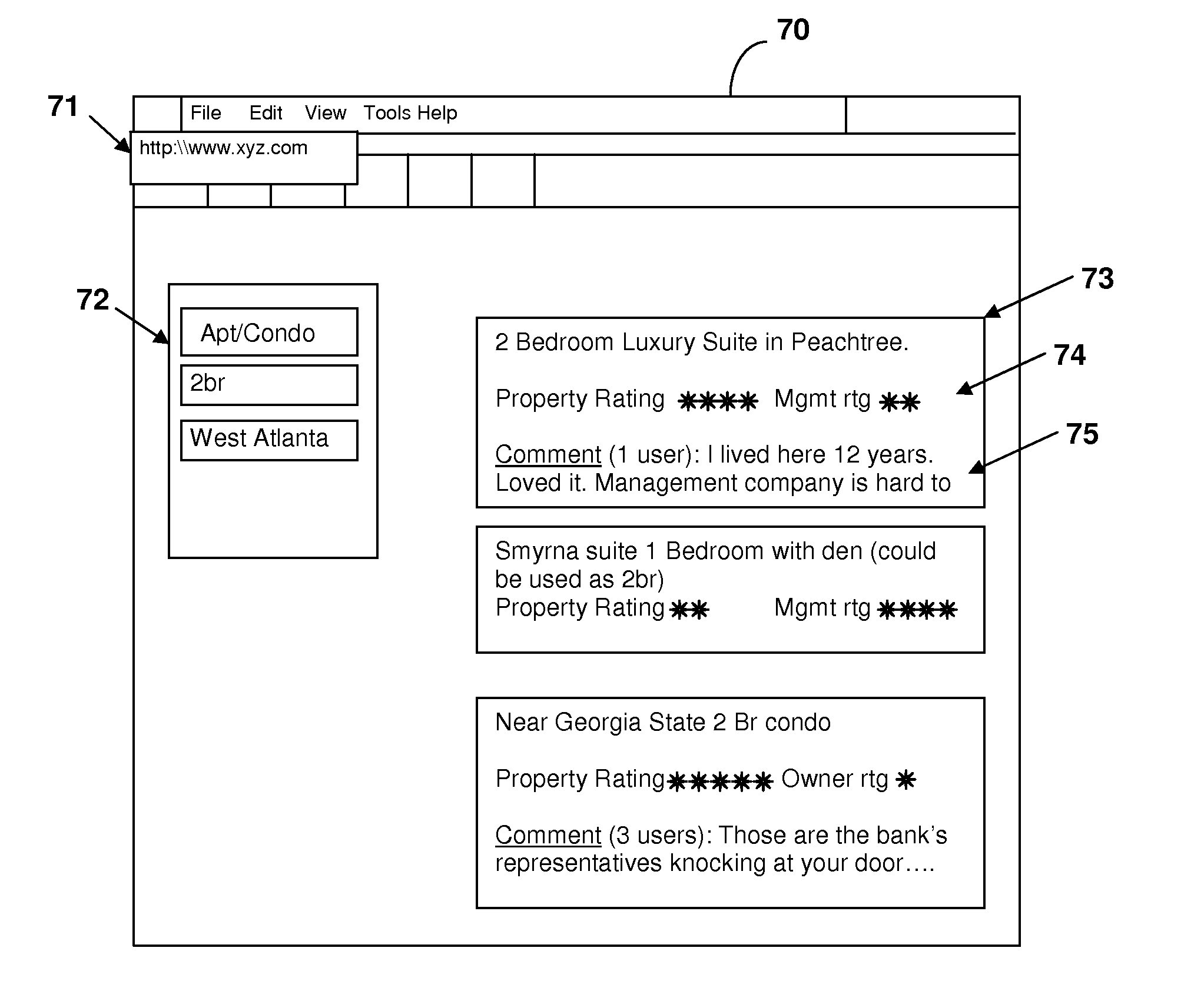
Internet portal for mortgagee/renter pass-through subscription
An internet portal for connecting prospective renters and property owners / managers provides features for further managing an on-going relationship between renters, property owners / managers and other parties such as mortagees. An agreement may be entered into via the portal that arranges rent on a property to be paid in a pass-through arrangement directly to the mortgagee, avoiding potential foreclosures of which a renter might be otherwise unaware. A rating system is provided by which performance of renters and the performance of the property owners / managers may be evaluated during the relationship and used to inform others as historical and comparative data.
Owner:ENM HLDG
Method and system for providing real-time clinical trial enrollment data
ActiveUS20070250779A1Data processing applicationsComputer-assisted medical data acquisitionTrial protocolCentral database
A method and system for enabling display of real-time clinical trial enrollment data. A set of computer forms corresponding to an application enable administrative personnel to define a plurality of clinical trial parameters, including trial protocols, clinical sites, and optional regions. As the data is entered, it is stored in a central database, typically through a dedicated connection between a client running the application and the database. Software and infrastructure for supporting an Internet web portal is also provided, whereby the web portal enables clinical site personnel to enter subject enrollment data that is stored in the database as it is entered (i.e., in real-time). Various charts pertaining to the subject enrollment data may then be generated, including subject status charts and subject enrollment rate charts. In general, the charts may be aggregated across individual sites, regions, and all sites corresponding to a given protocol.
Owner:SIEBEL SYST INC
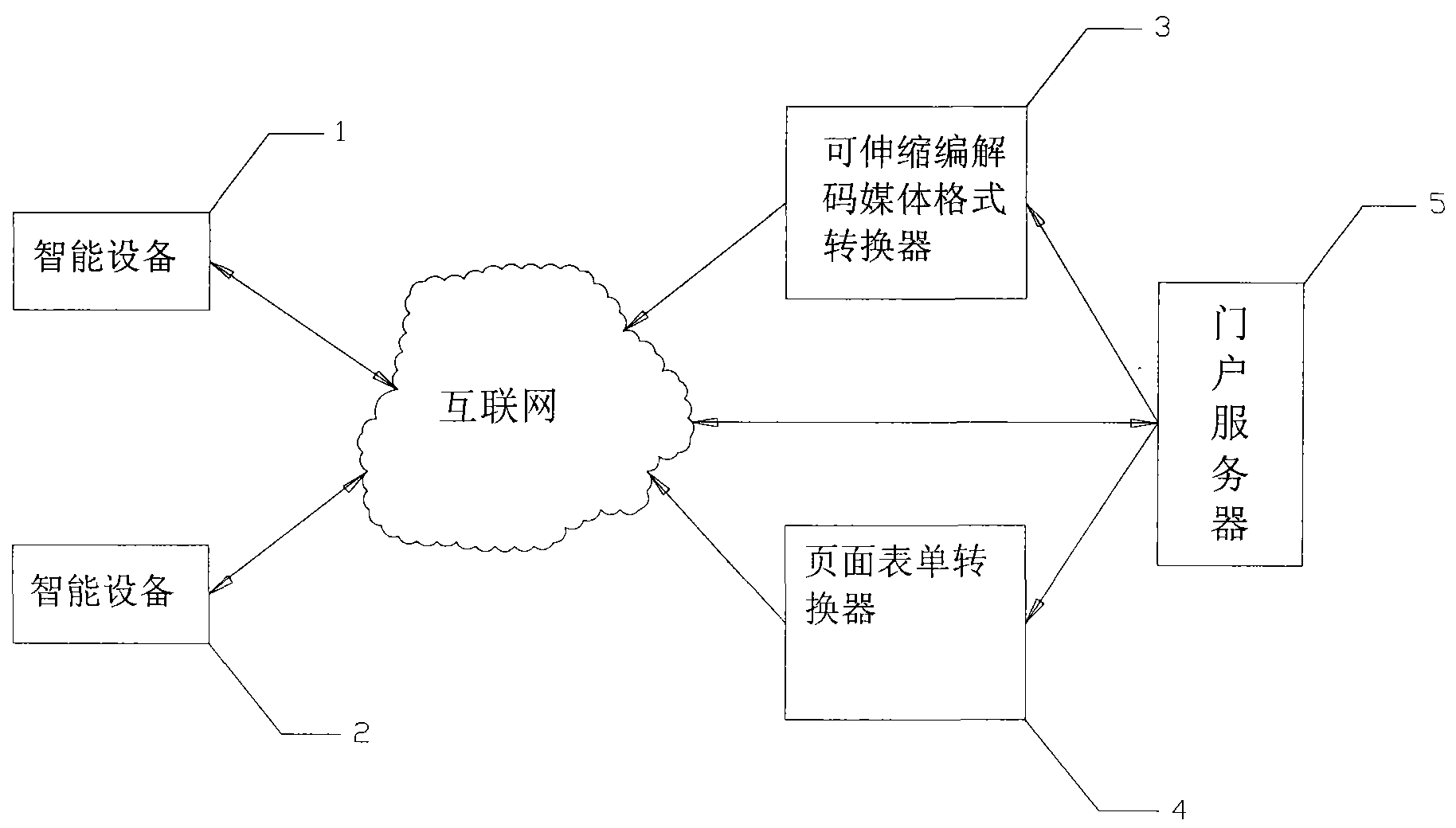
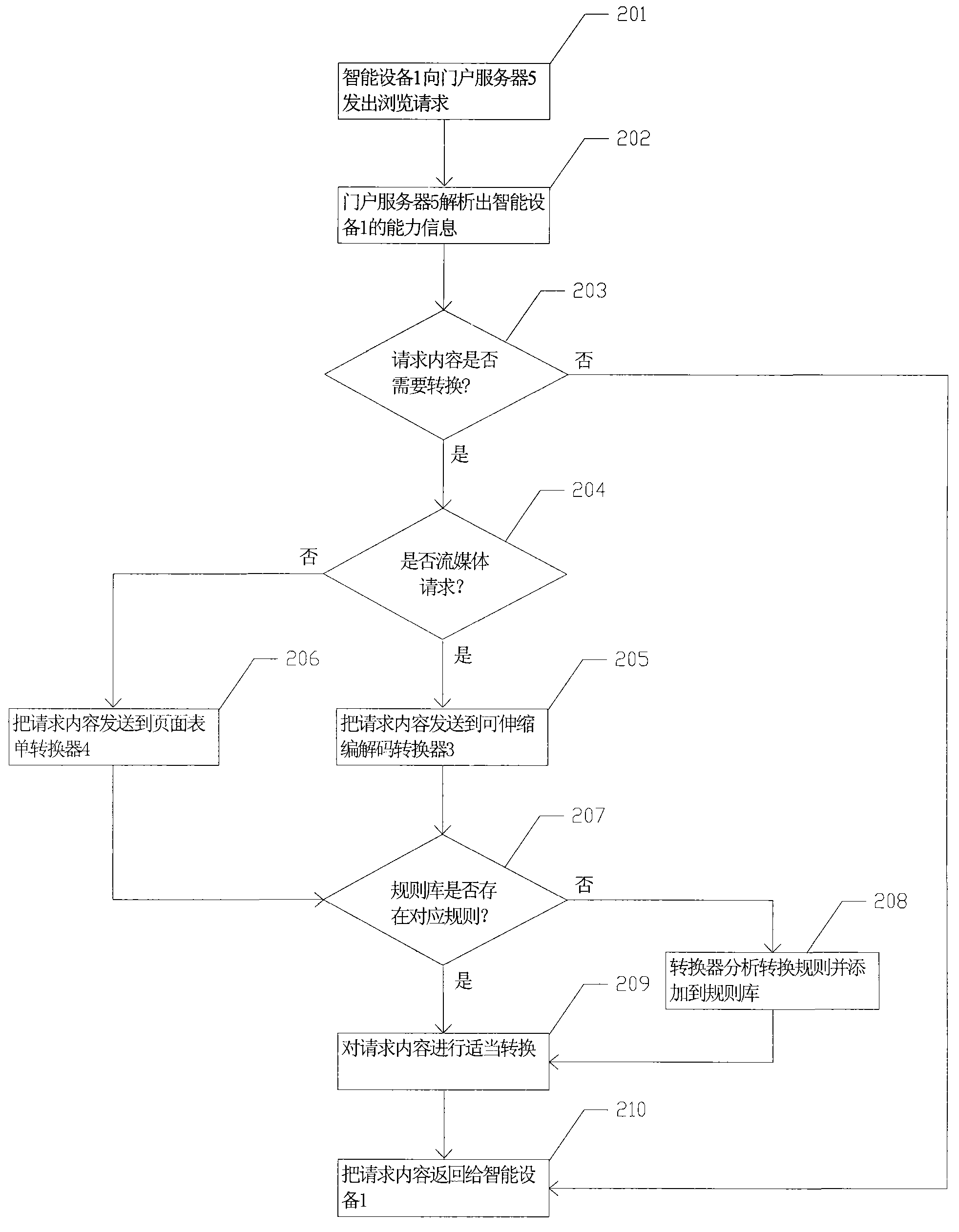
Internet portal service system and management method thereof
InactiveCN101640664AAchieving processing powerData switching by path configurationReal time analysisIntelligent equipment
The invention discloses an Internet portal service system and a management method thereof. The system comprises an Internet portal and an application service converter. The management method comprisesthe following implementation steps that: an intelligent device sends browsing request to a portable server; after receiving the request, the portable server resolves capacity information of the intelligent device which sends the request; if the request content does not need to be converted, the portable server directly returns the request content to the intelligent device; otherwise, the portableserver forwards the content of the request and the capacity information of the intelligent device to the corresponding converter; the converter looks up a rule base according to the capacity information of the device, and carries out format conversion for the content in a proper mode after finding out the corresponding conversion rule; otherwise, the converter analyses the conversion rule of thedevice in real time, carries out format conversion for the request content in a proper mode, and dynamically adds the conversion rule to the rule base; and the converter returns the converted contentto the intelligent device.
Owner:TCL CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com