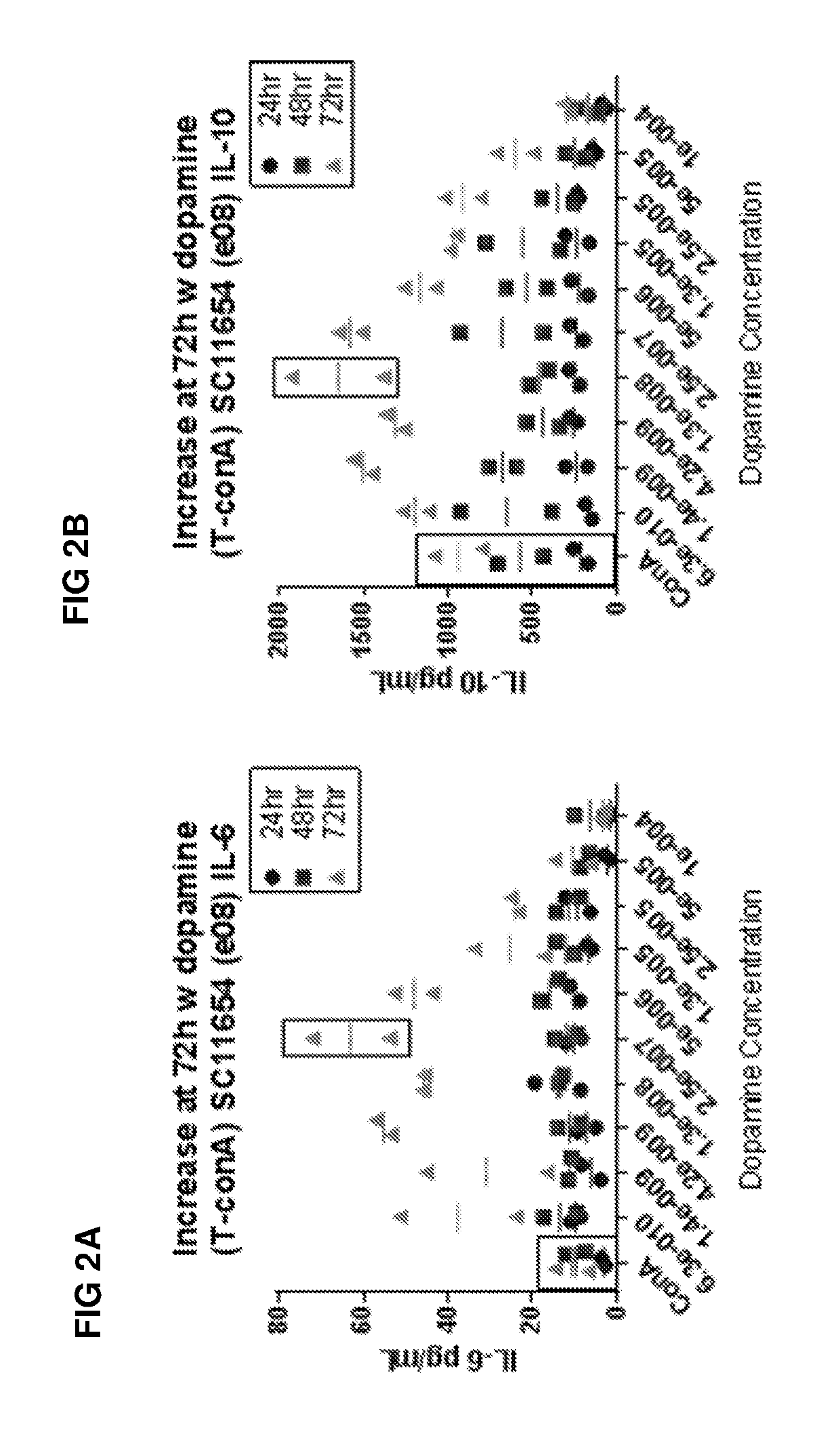
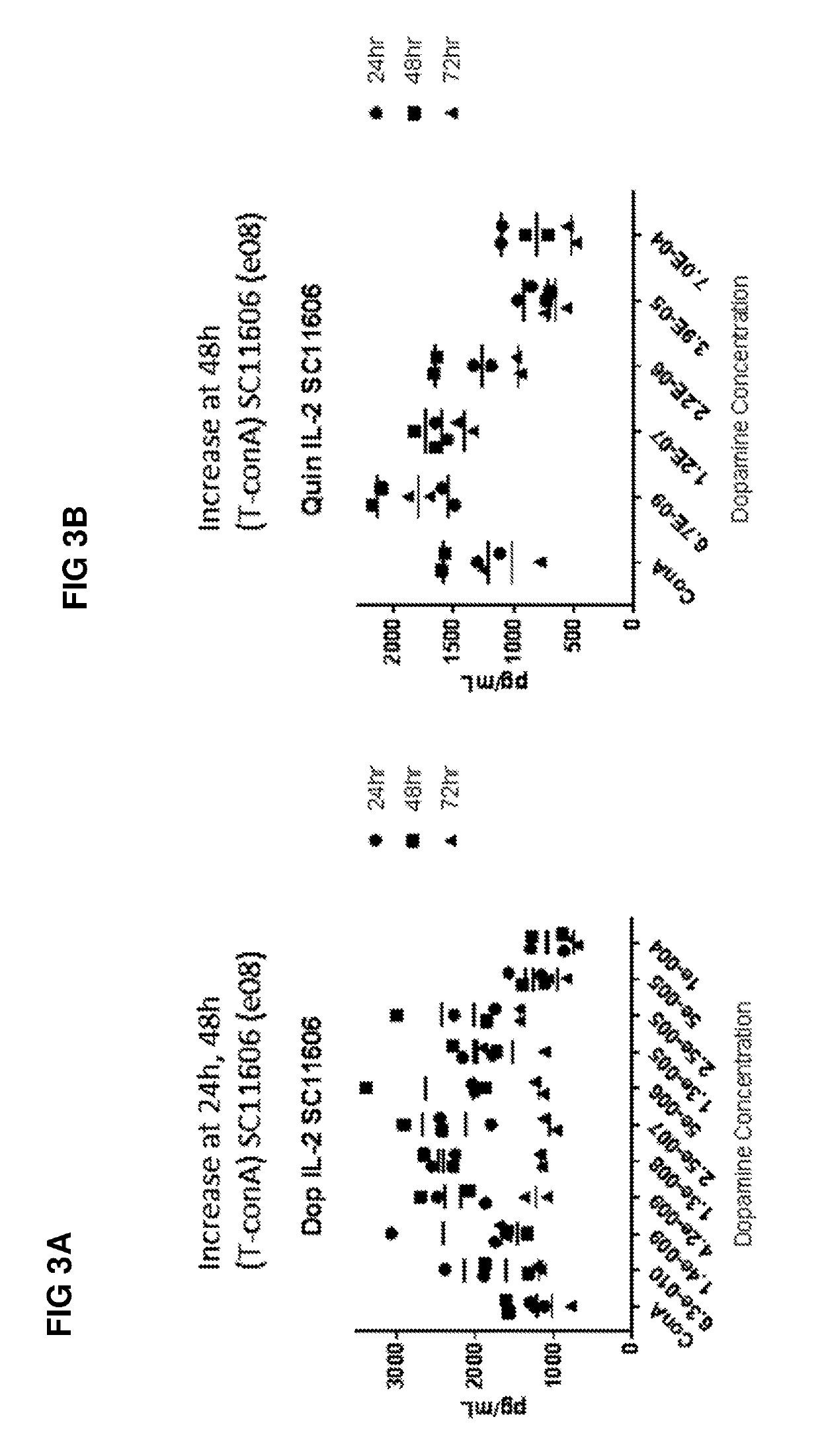
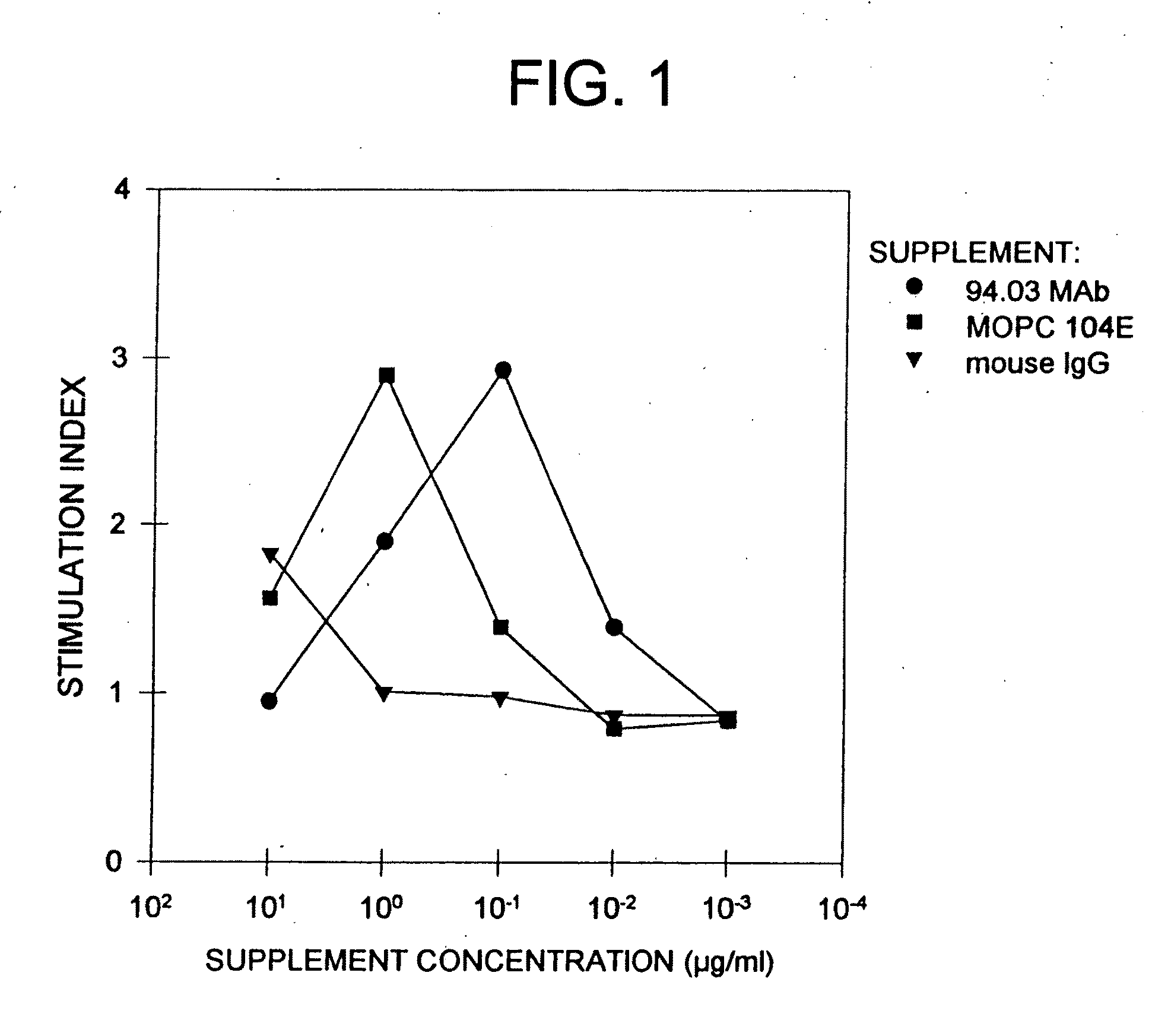
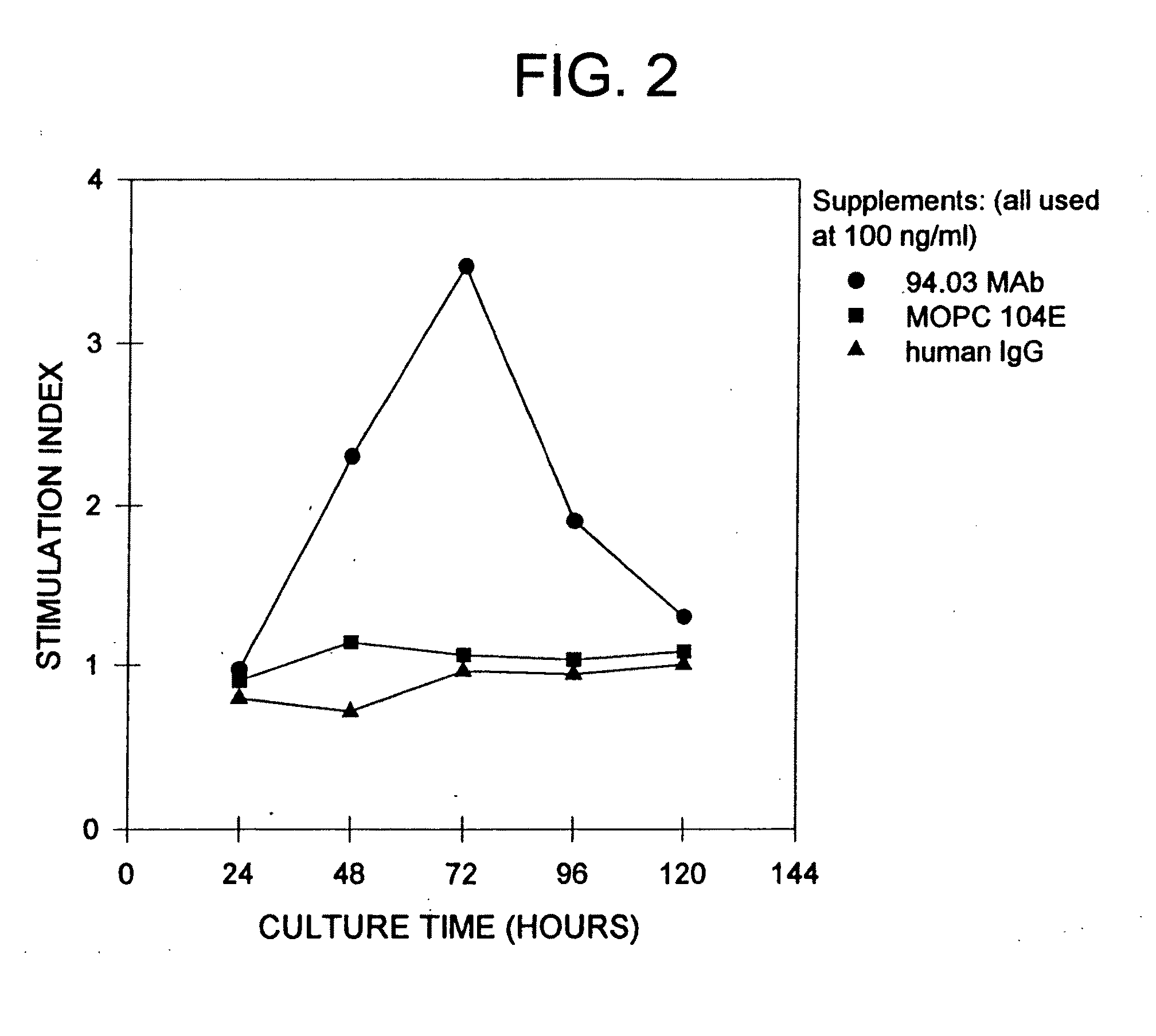
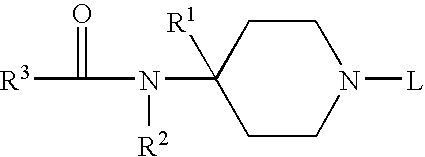Patents
Literature
46 results about "Neuroregulator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
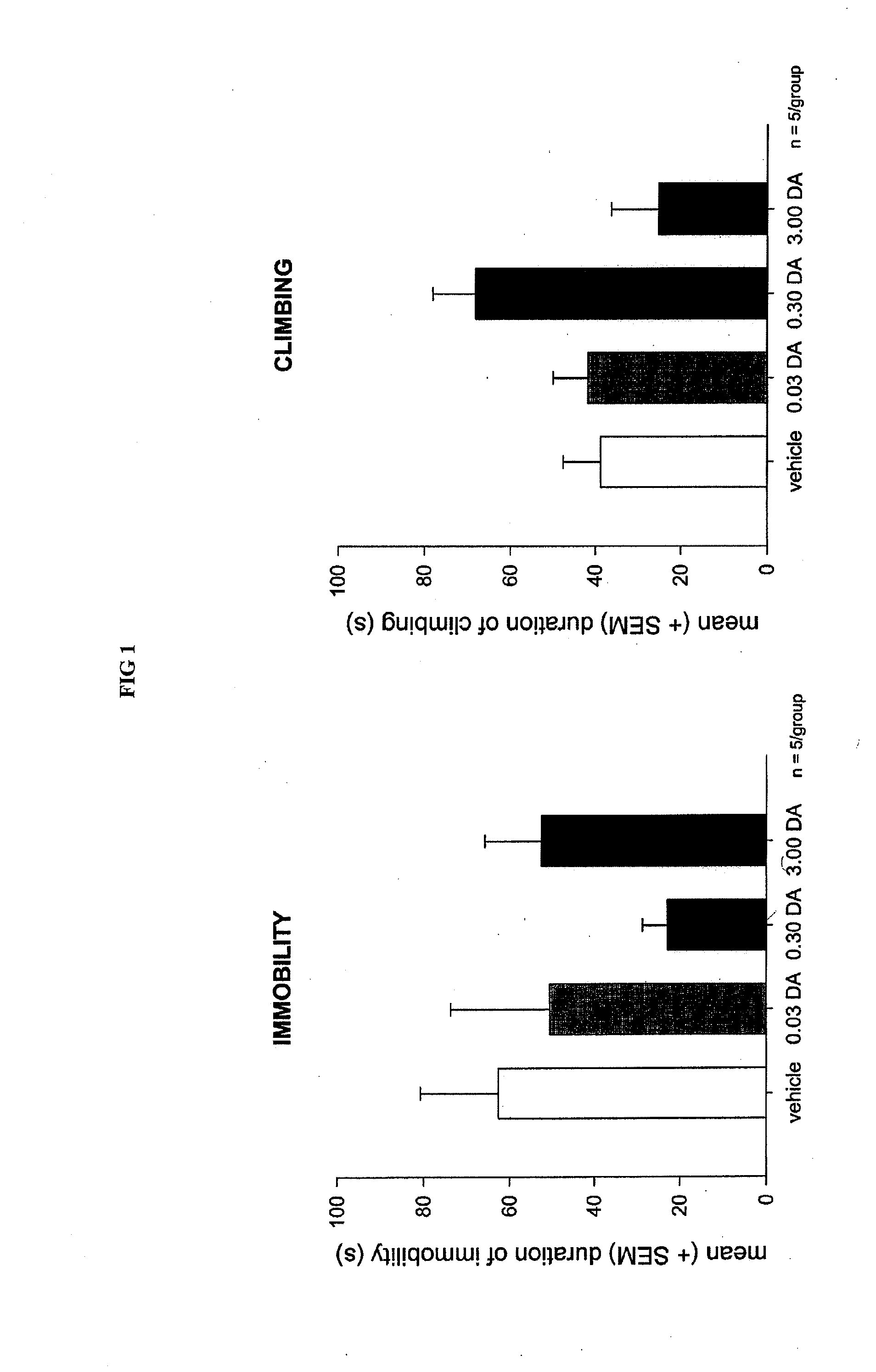
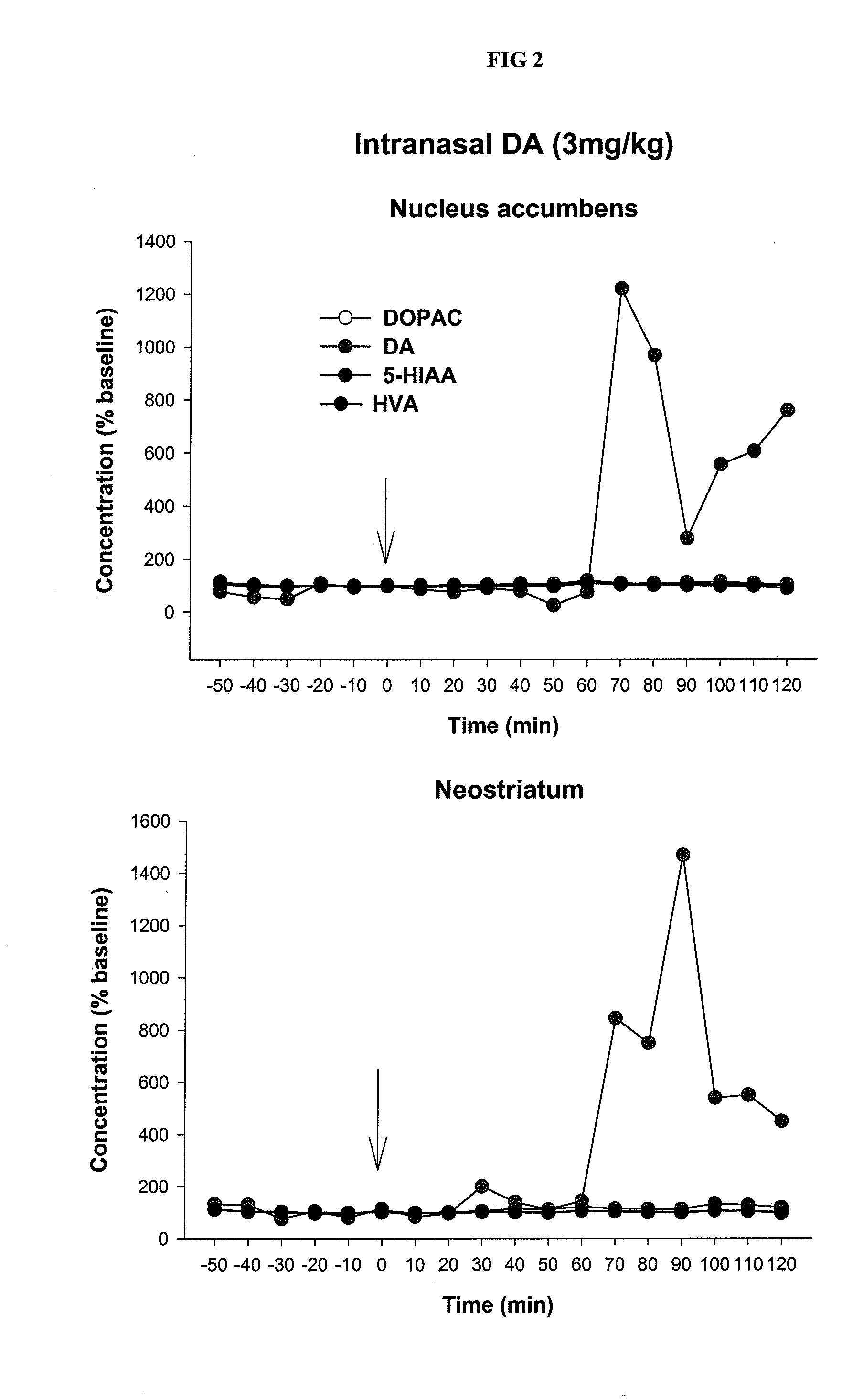
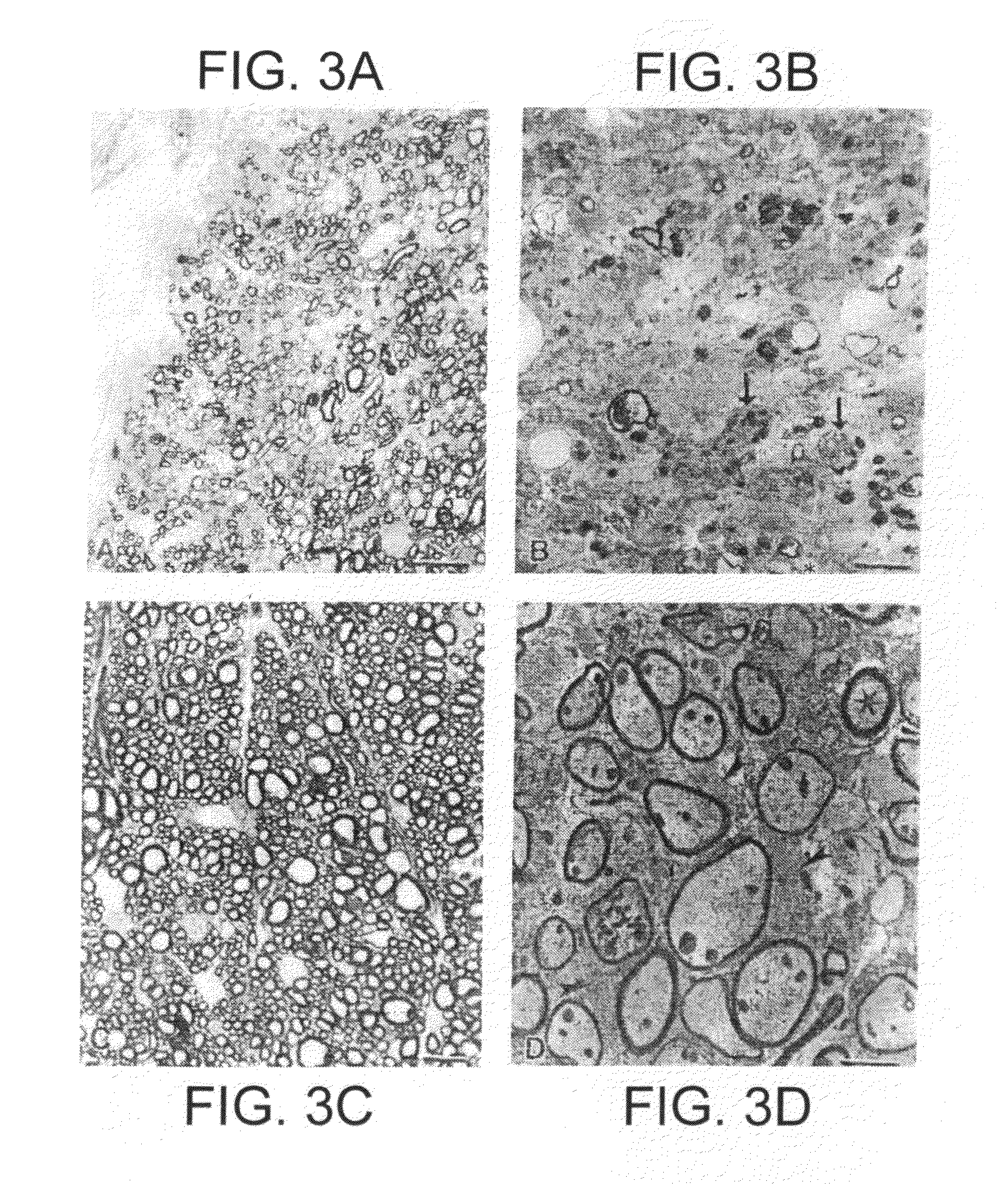
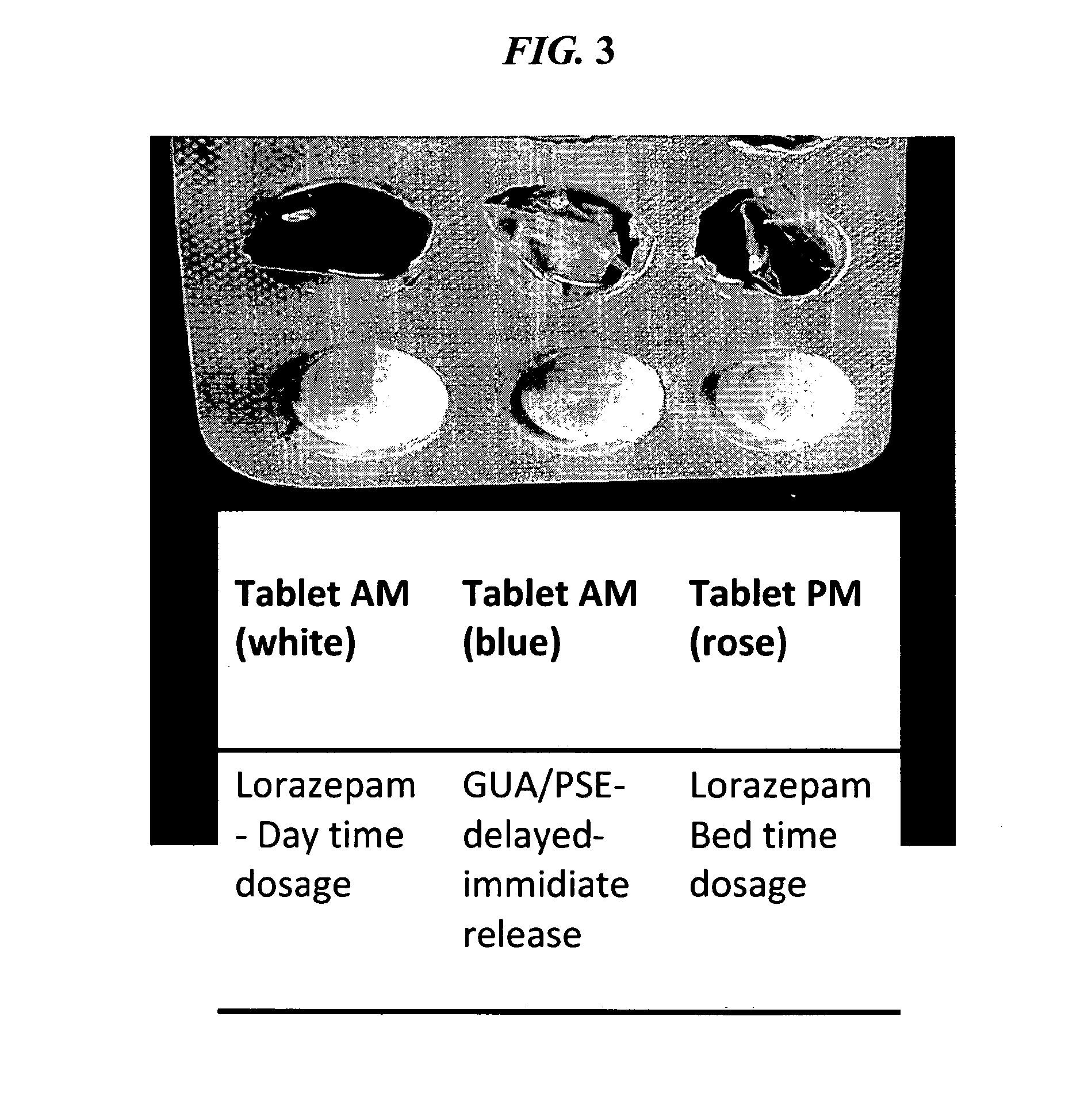
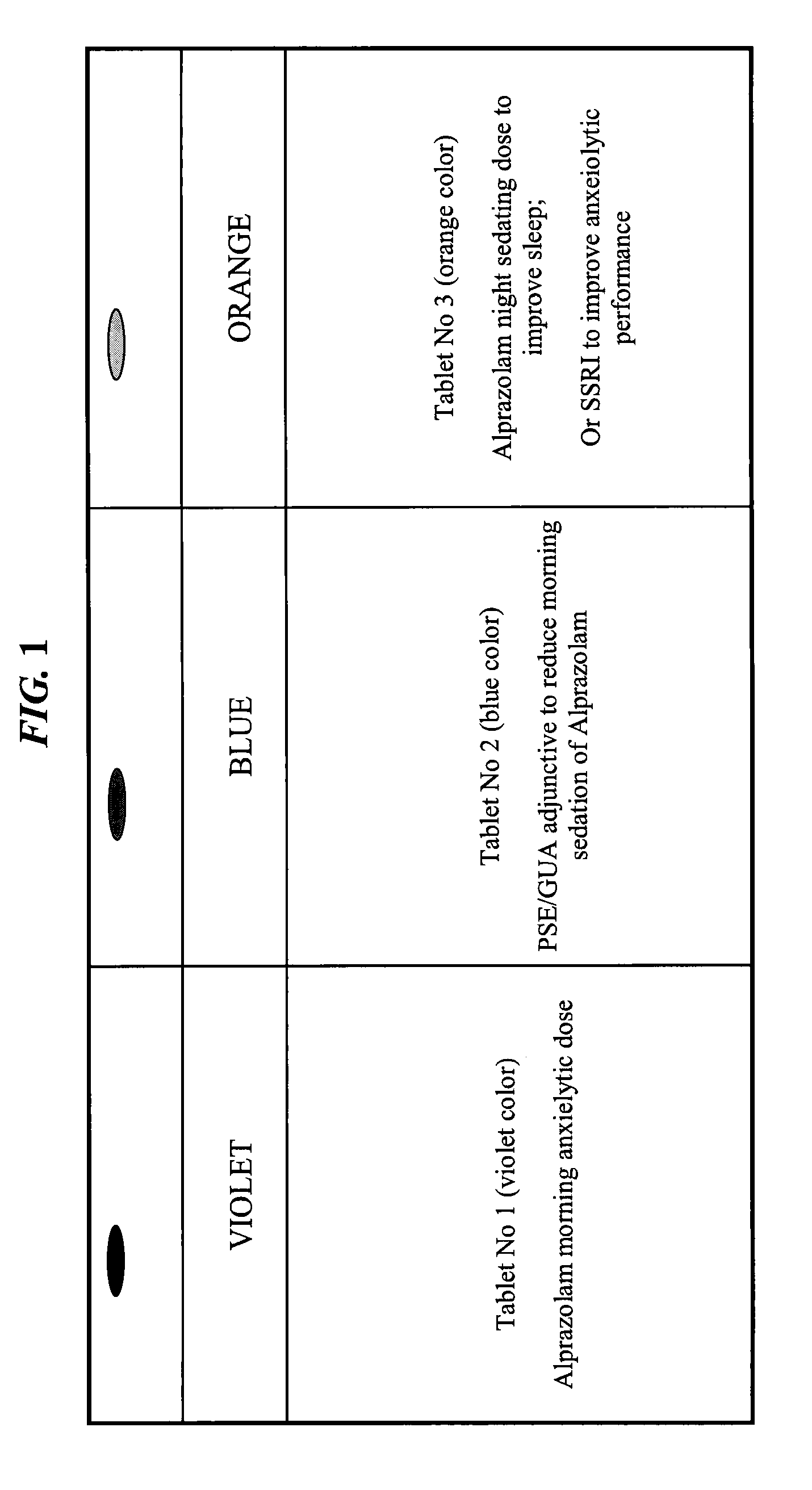
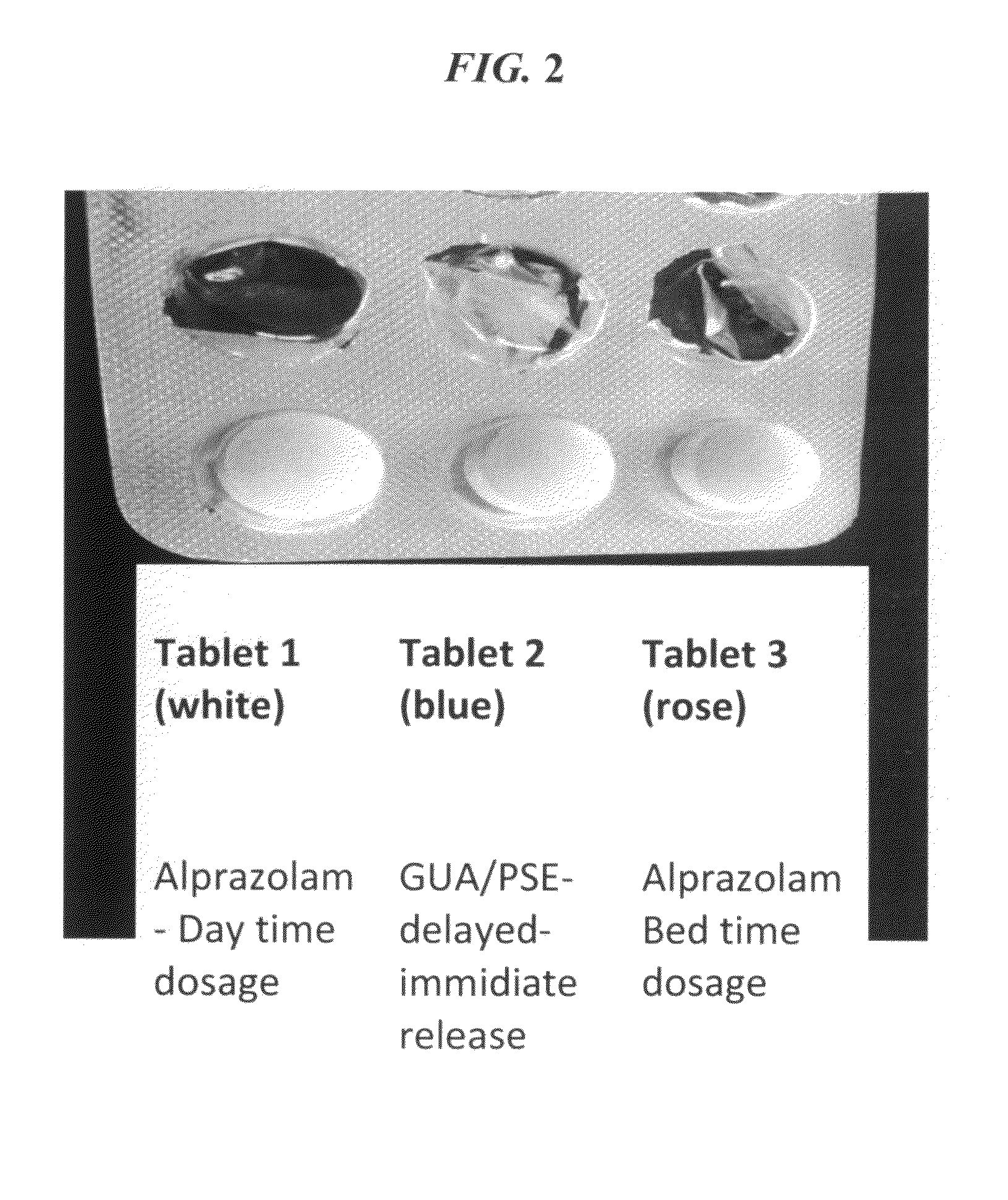
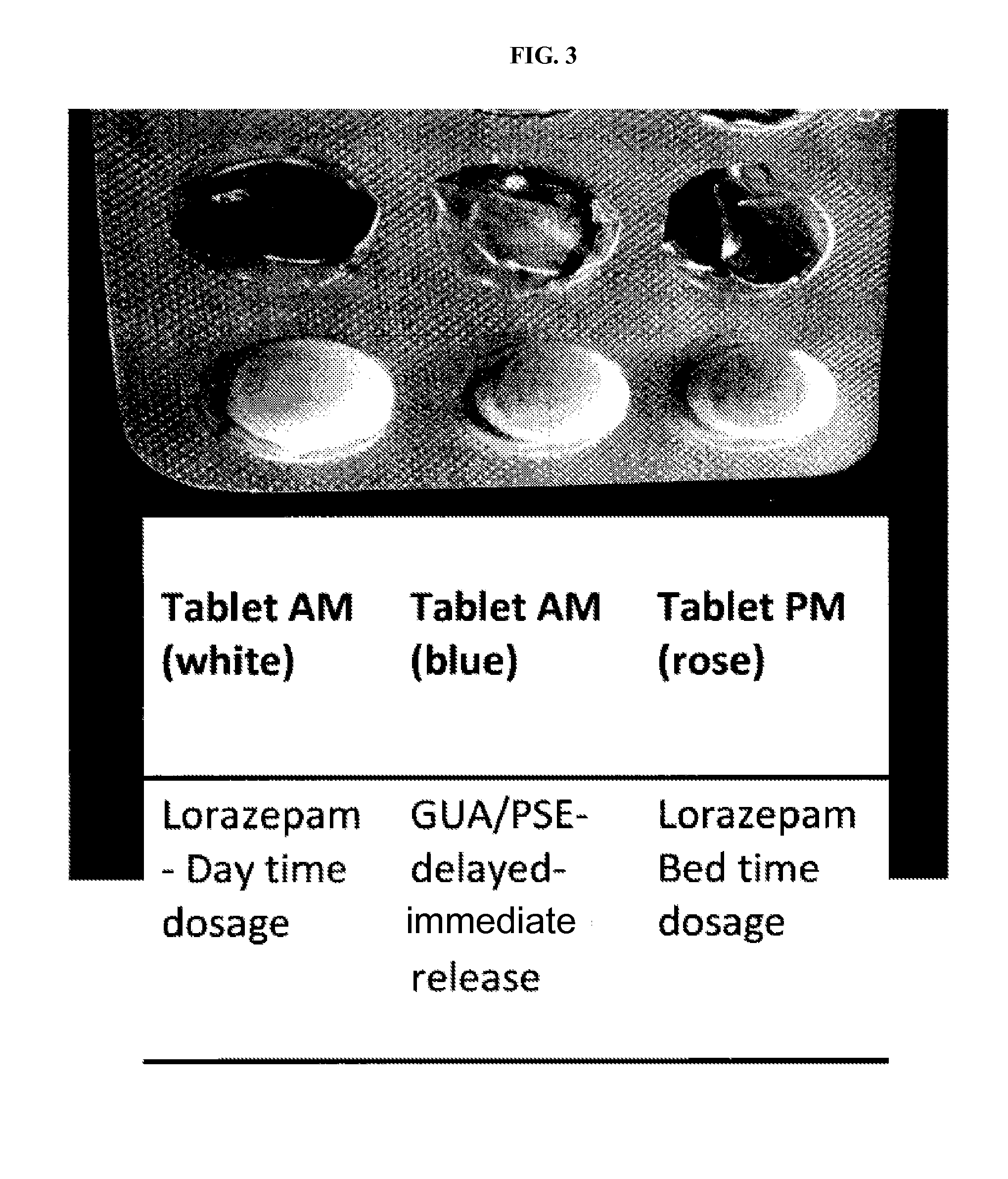
Serious adverse events in the ReCharge study were nausea, pain at the neuroregulator site, vomiting, and surgical complications; other adverse events were heartburn, problems swallowing, belching, mild nausea, and chest pain, the FDA noted.
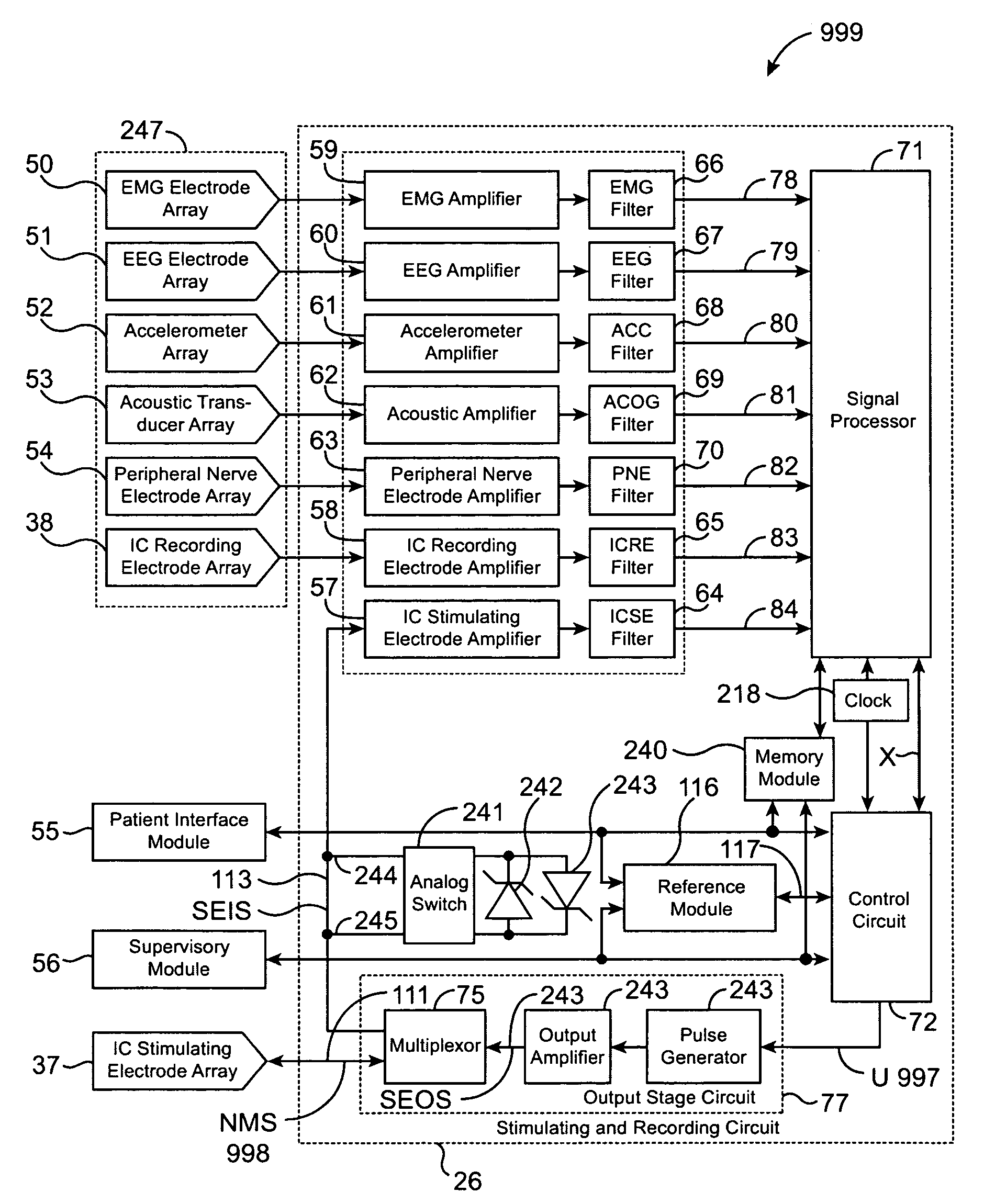
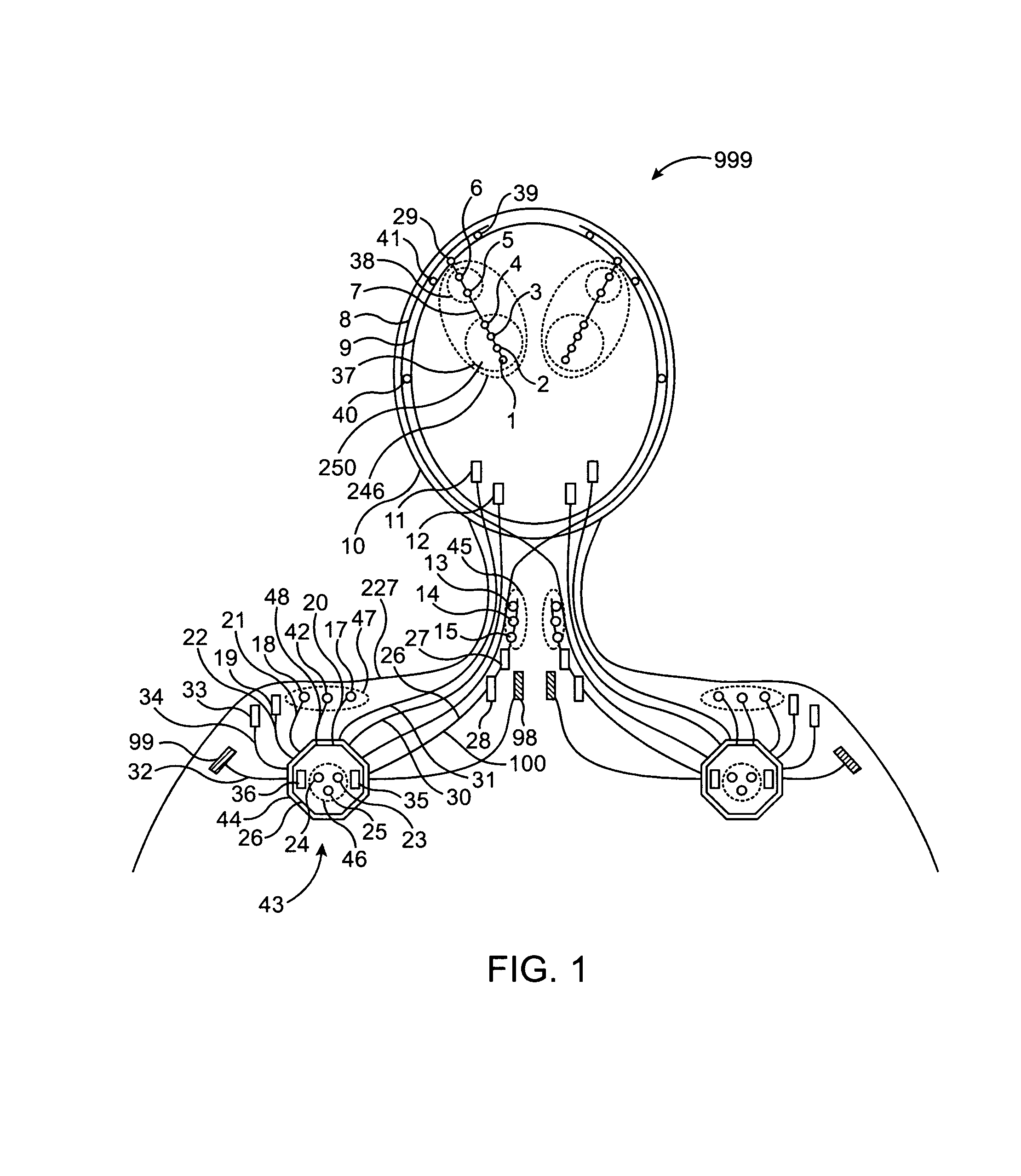
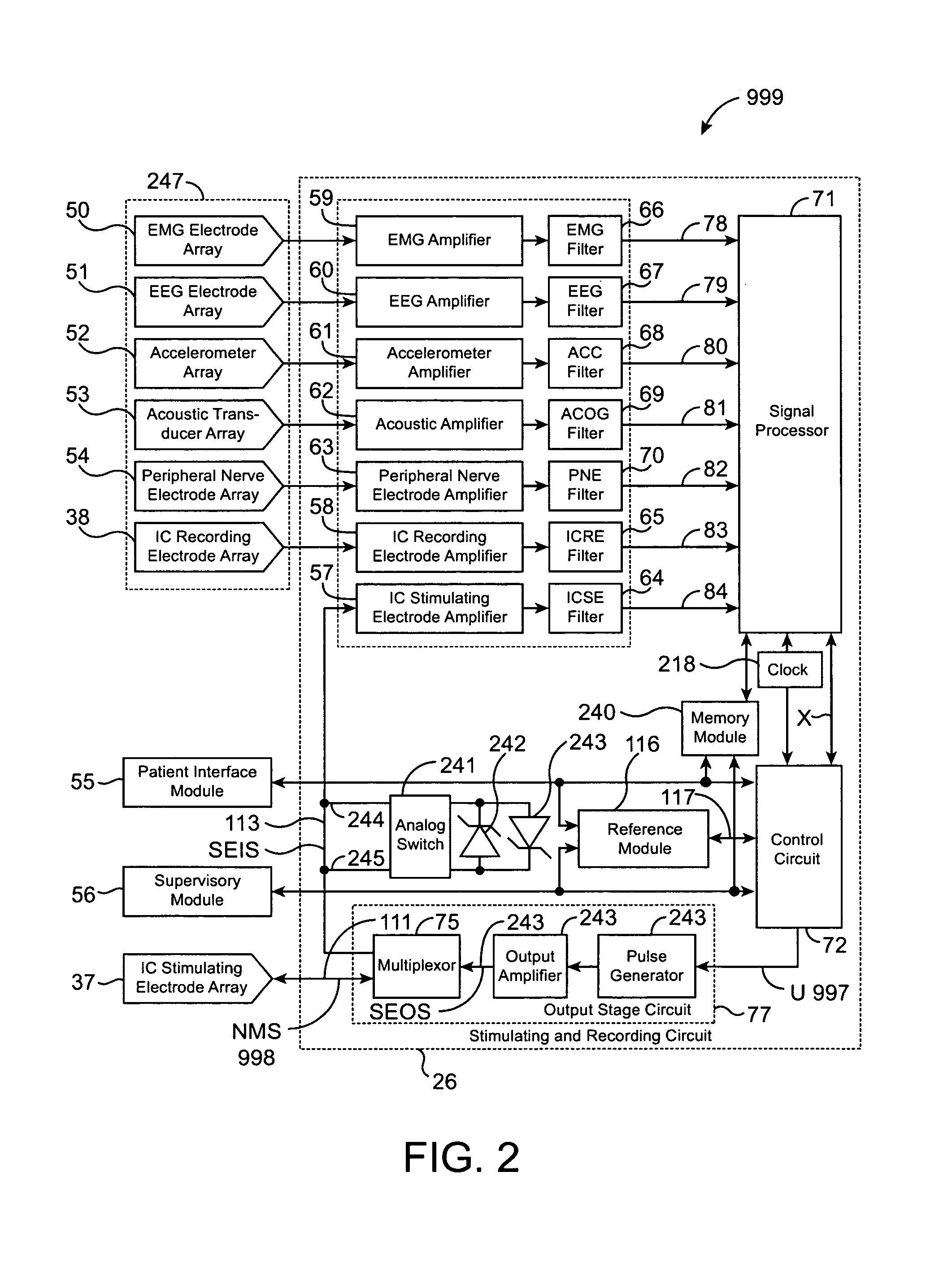
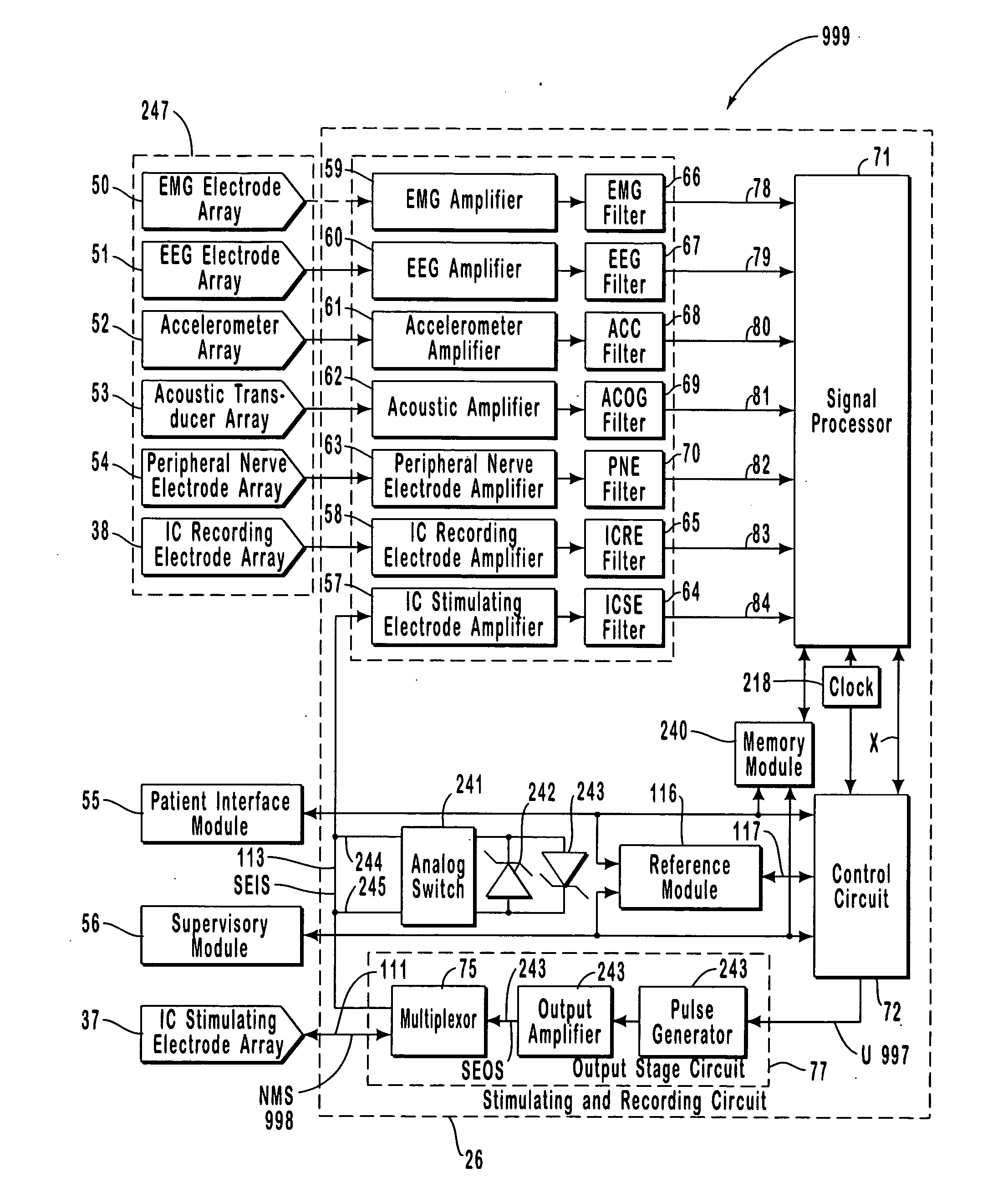
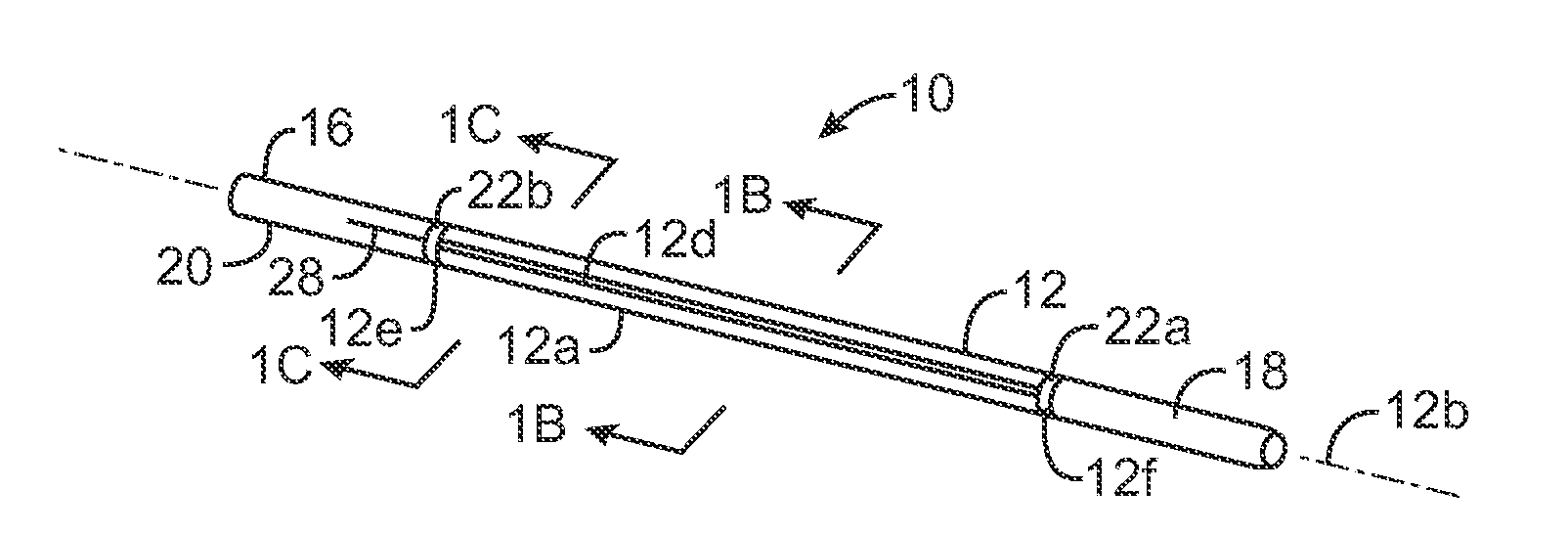
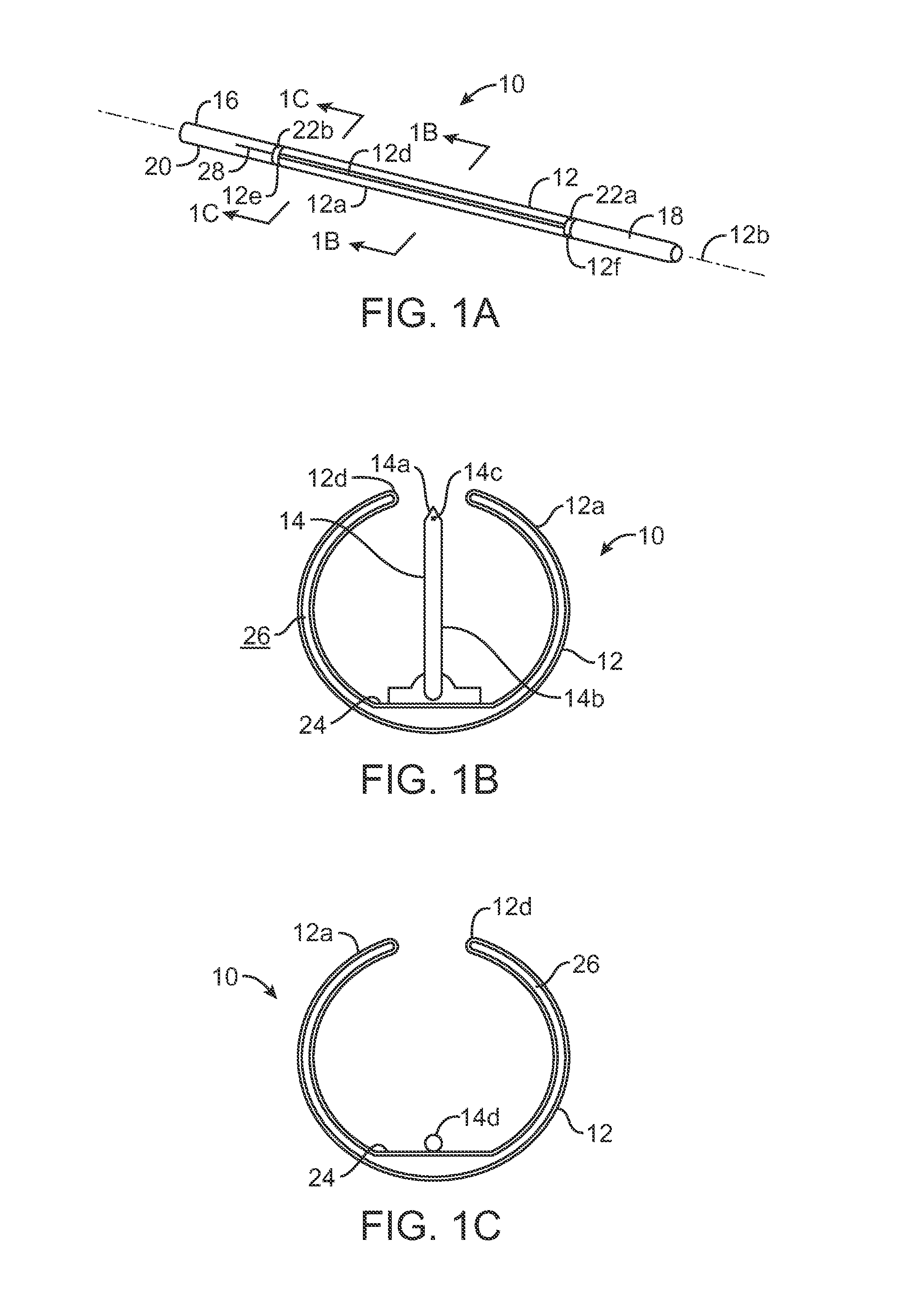
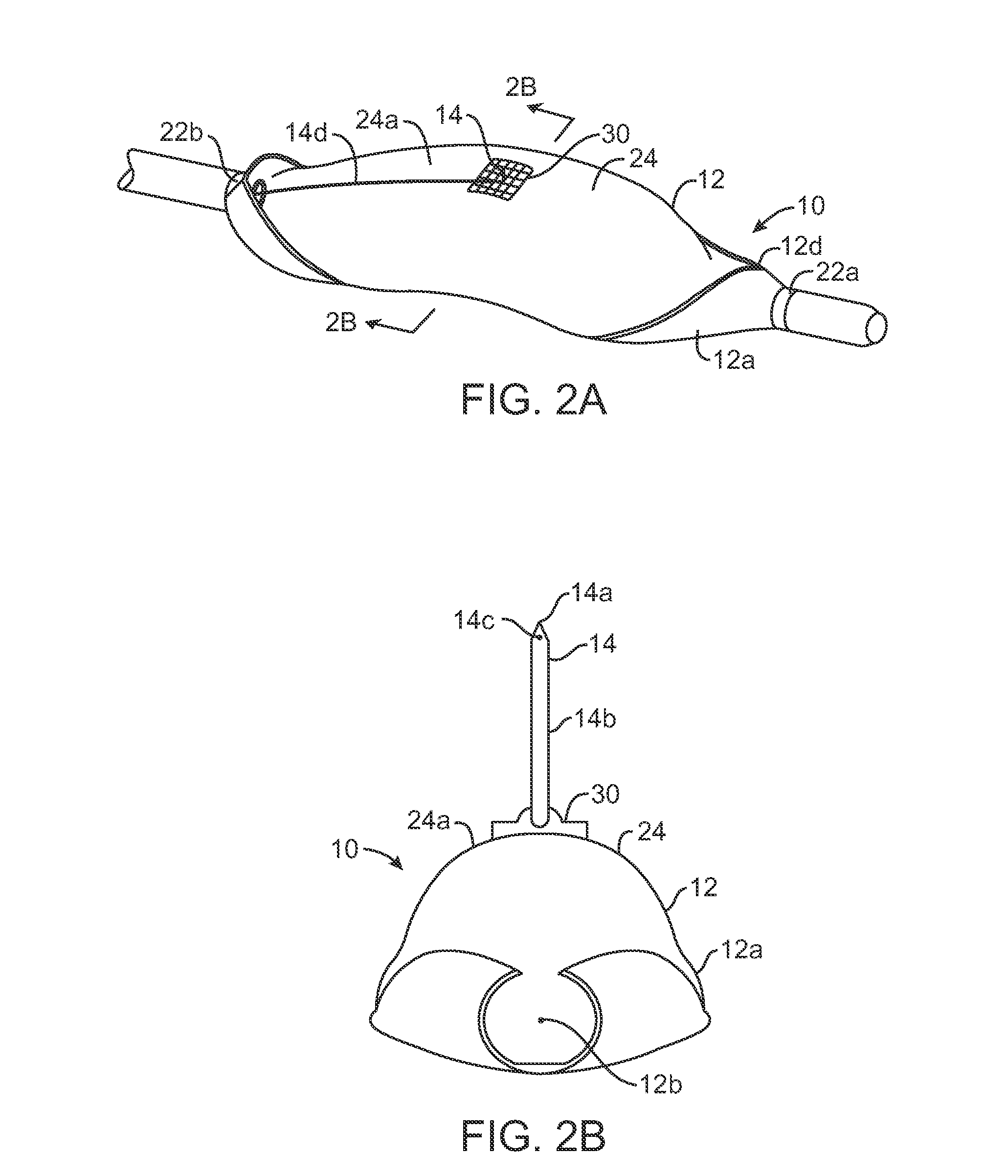
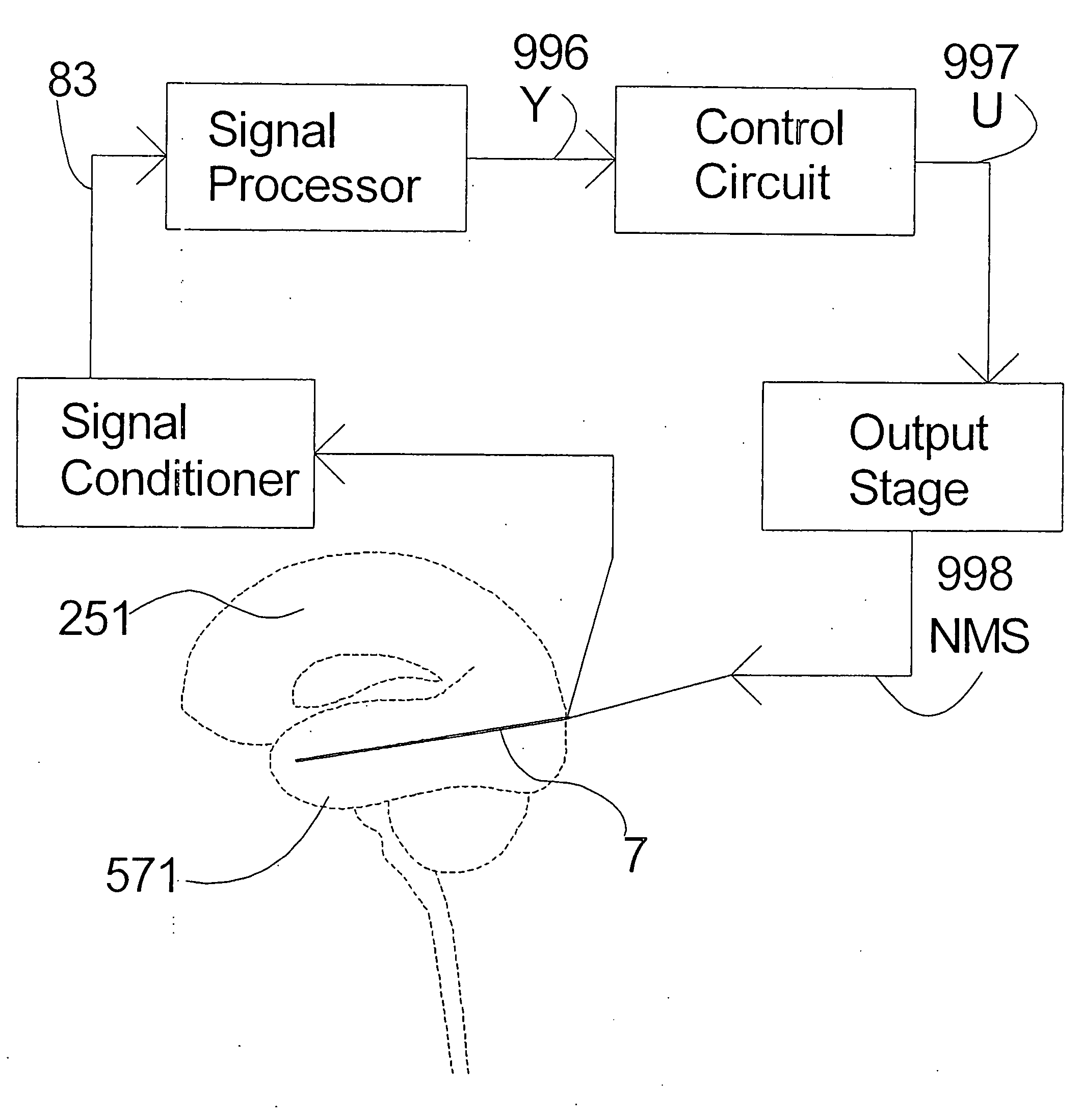
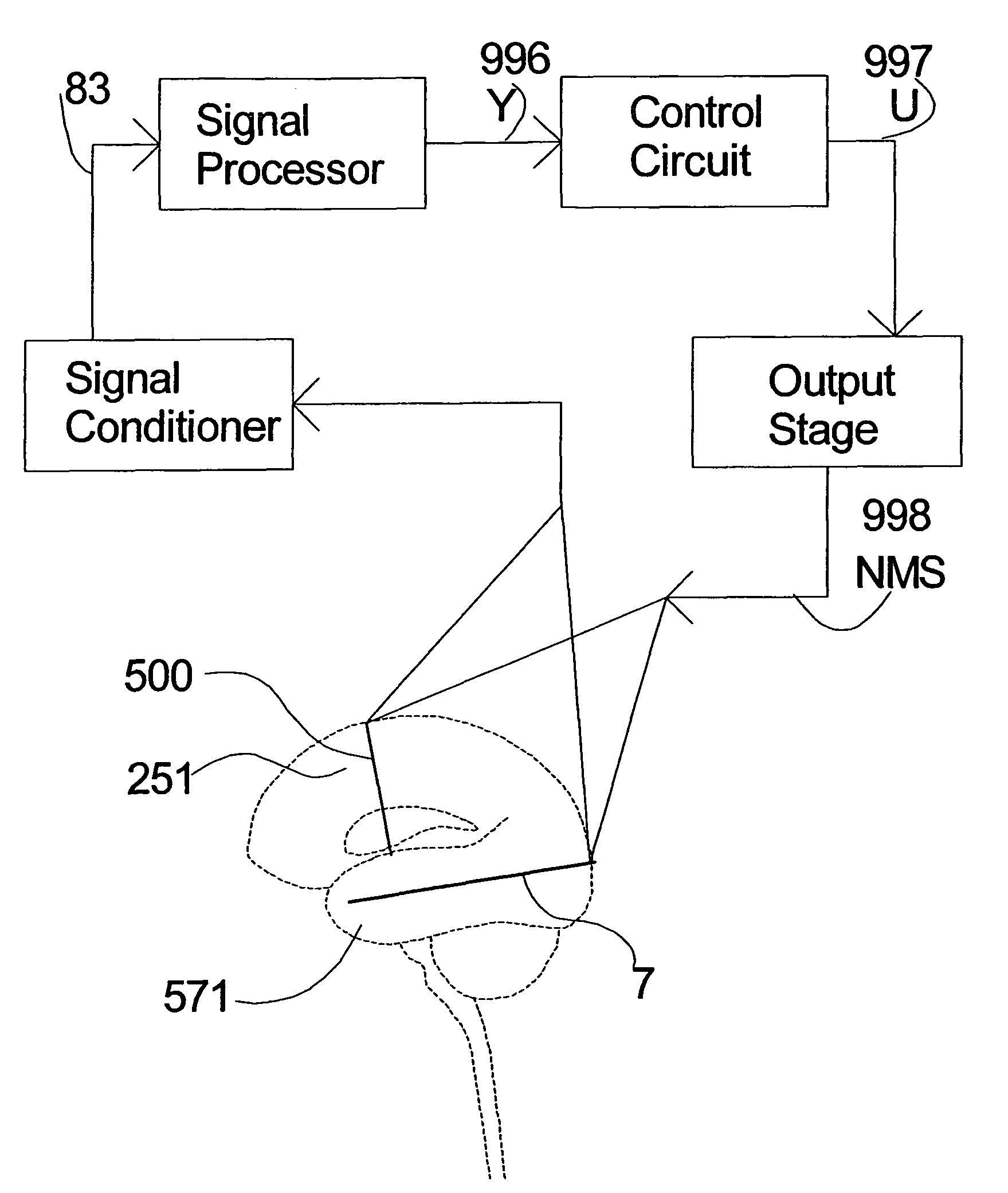
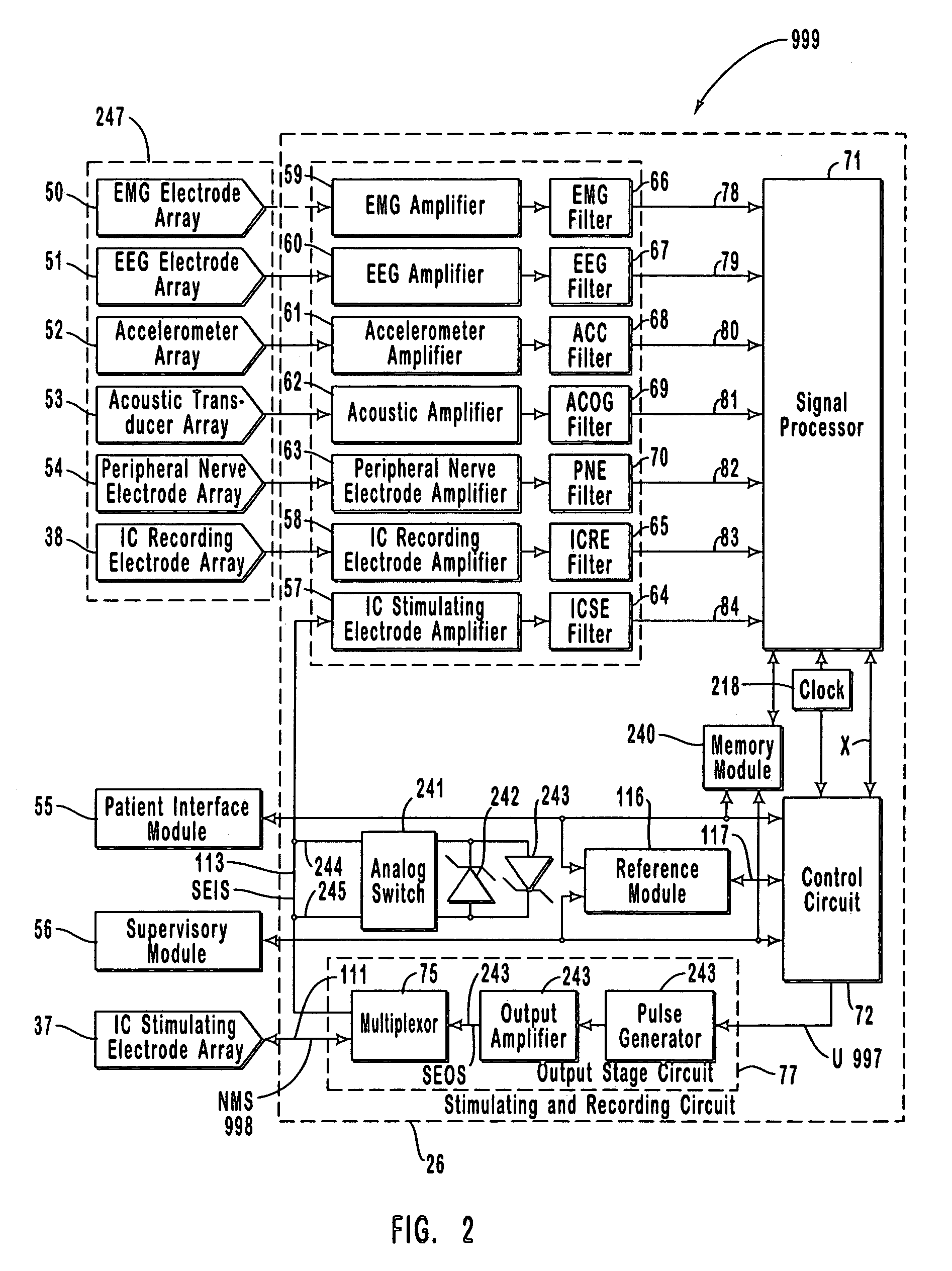
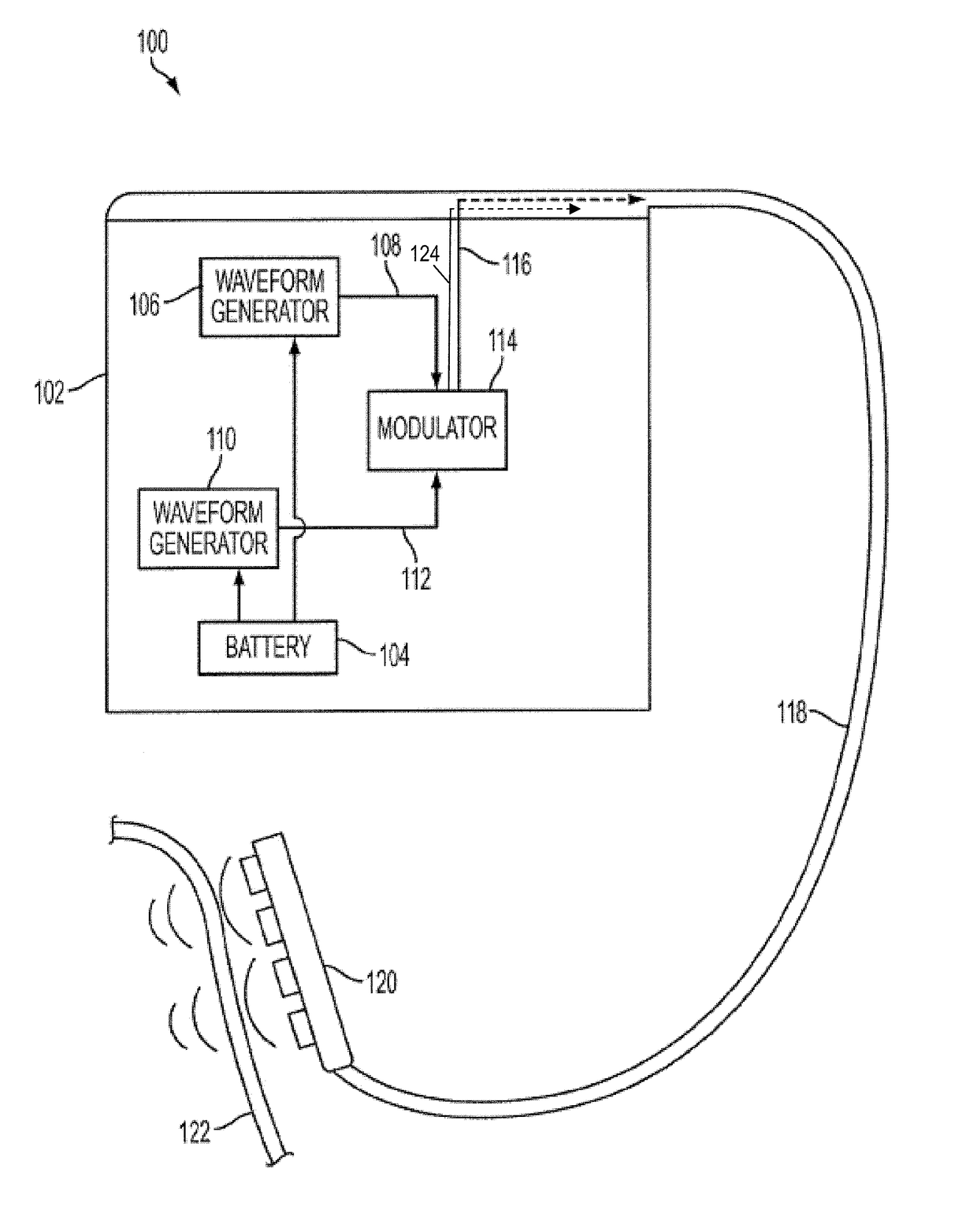
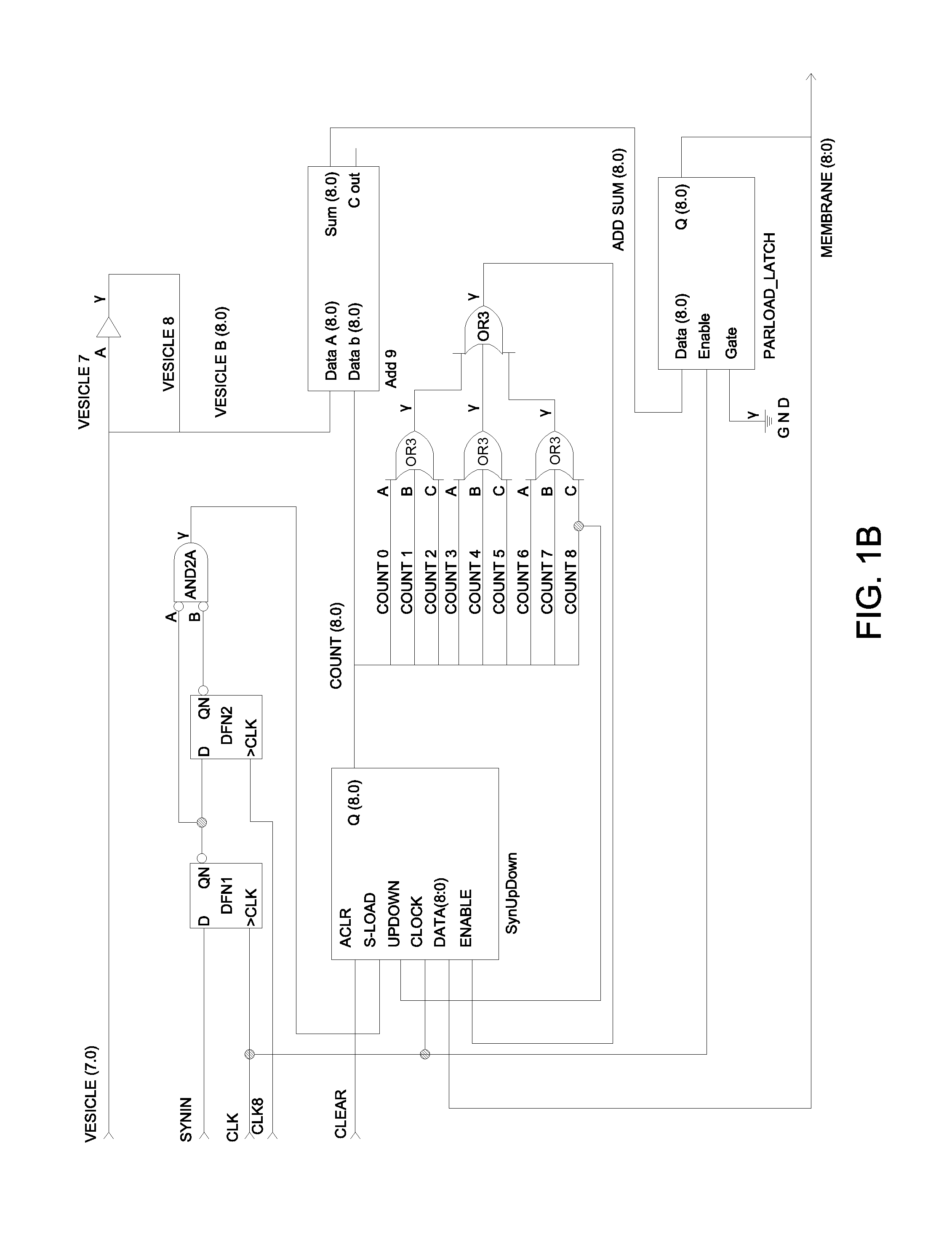
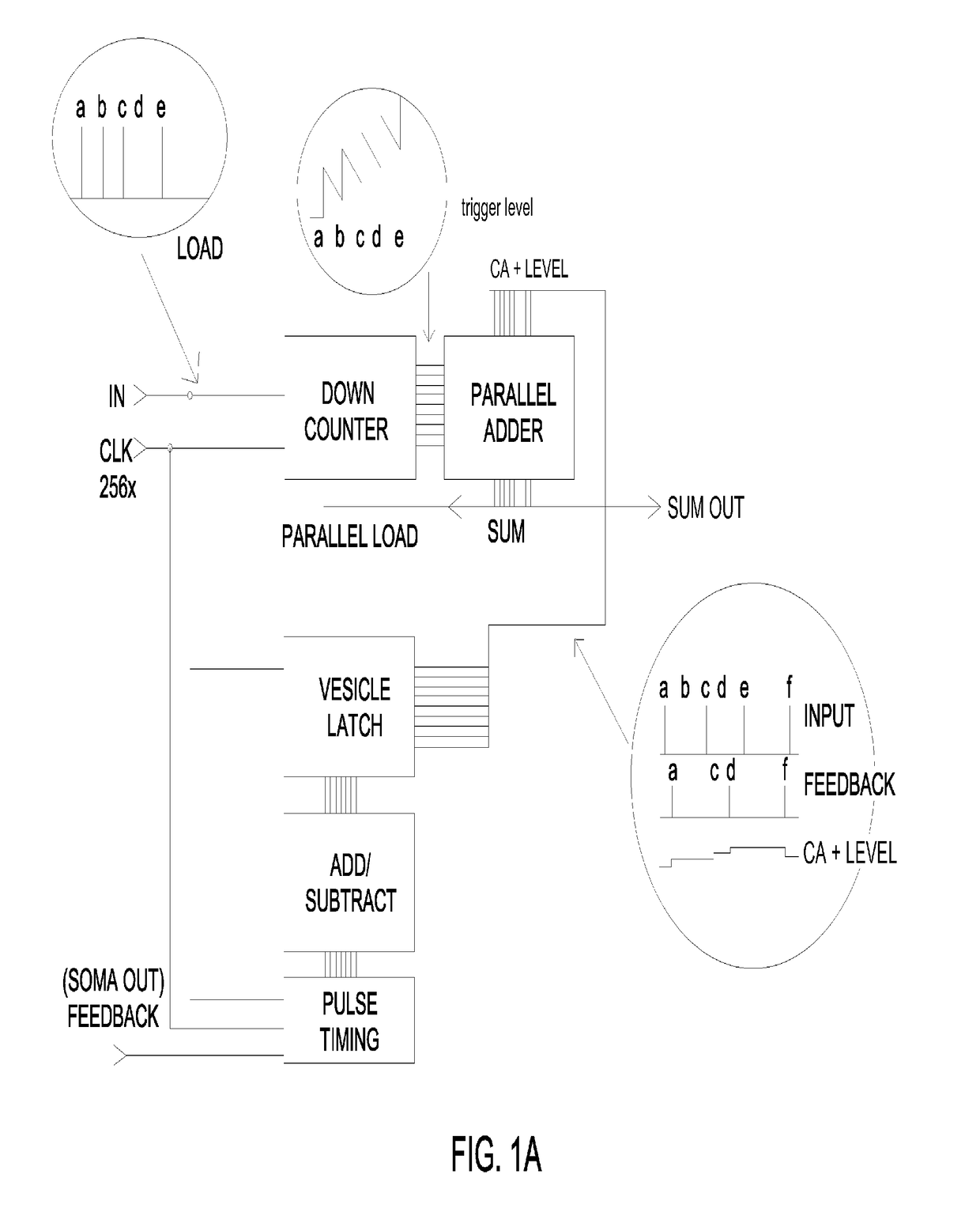
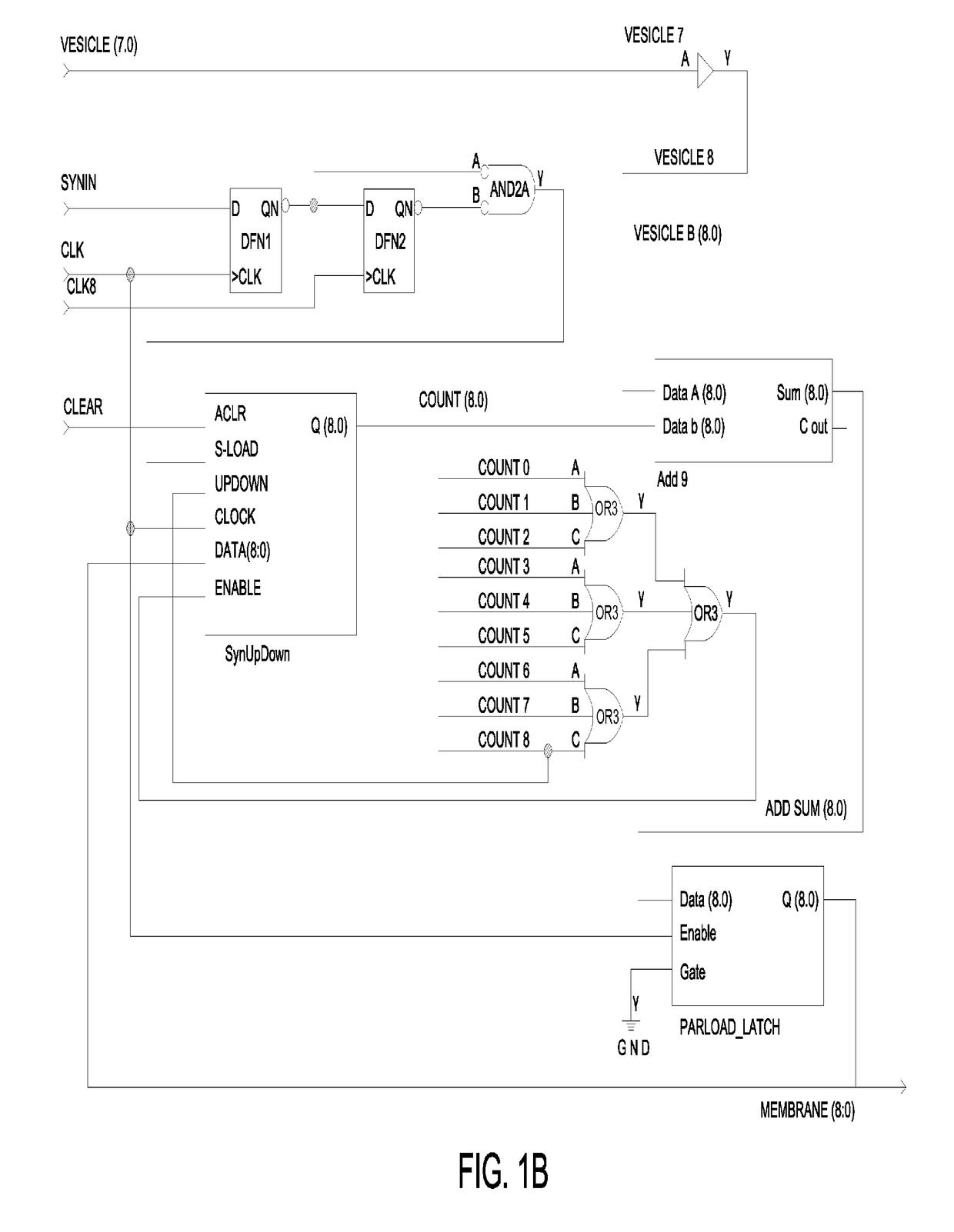
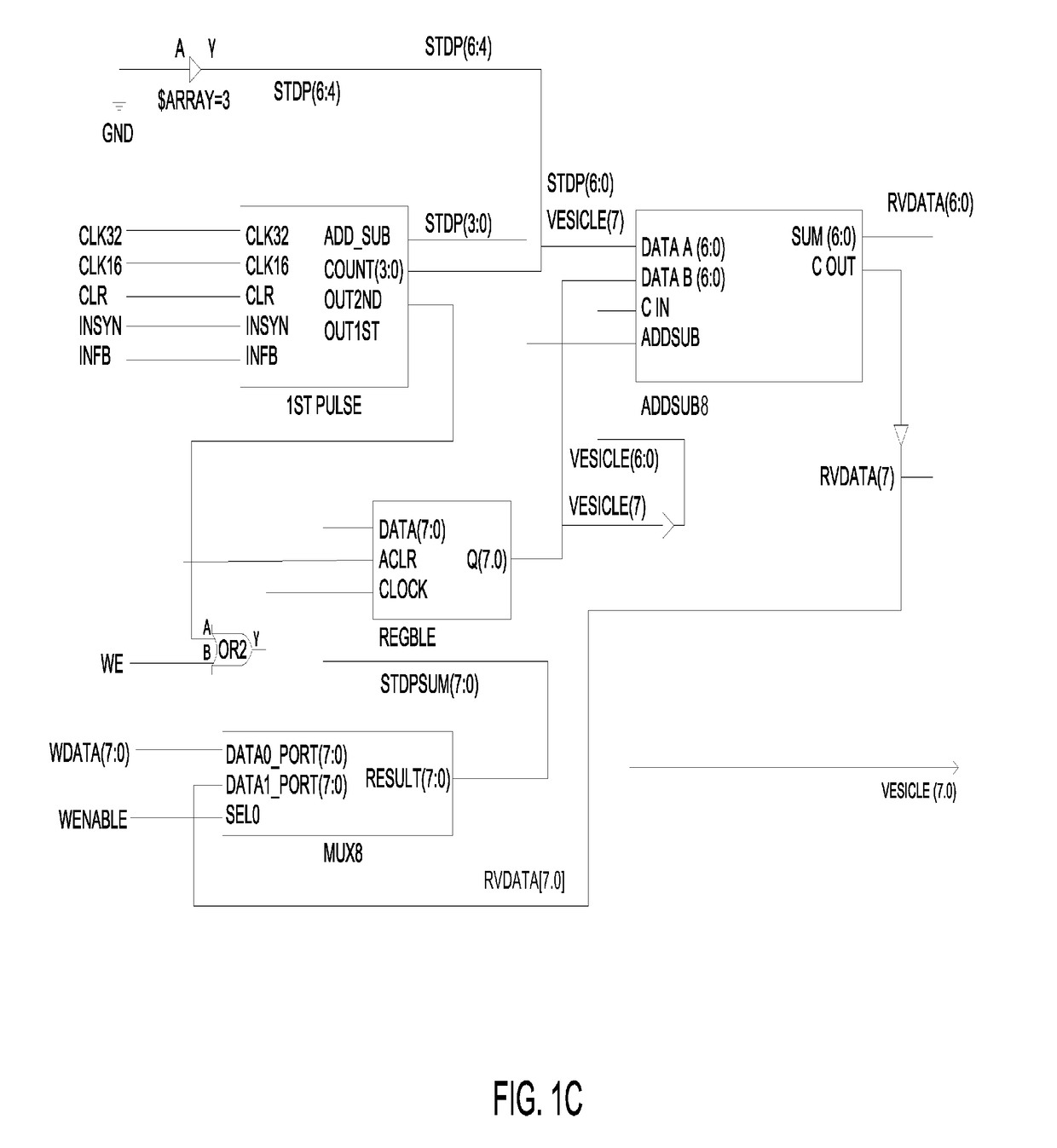
Closed-loop feedback-driven neuromodulation
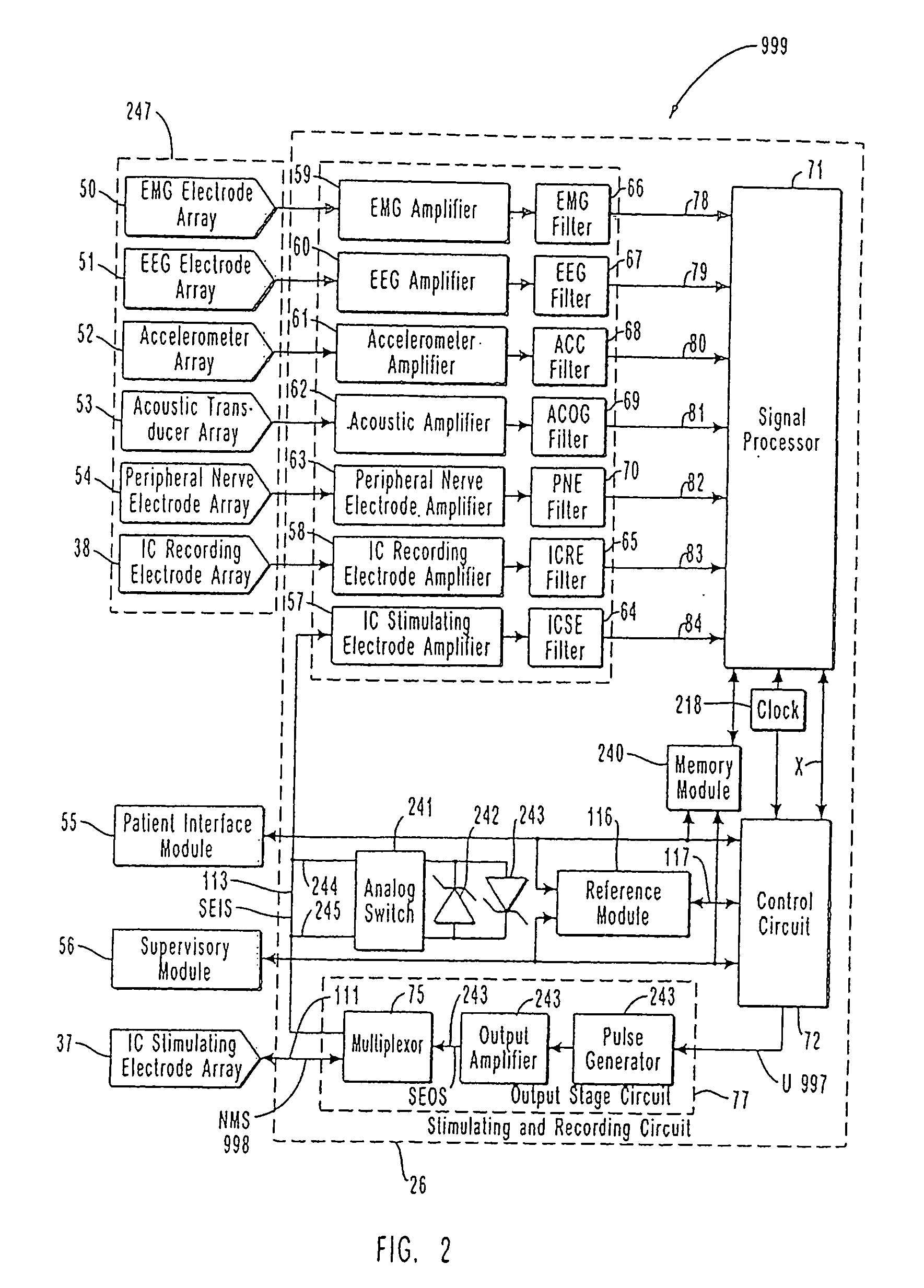
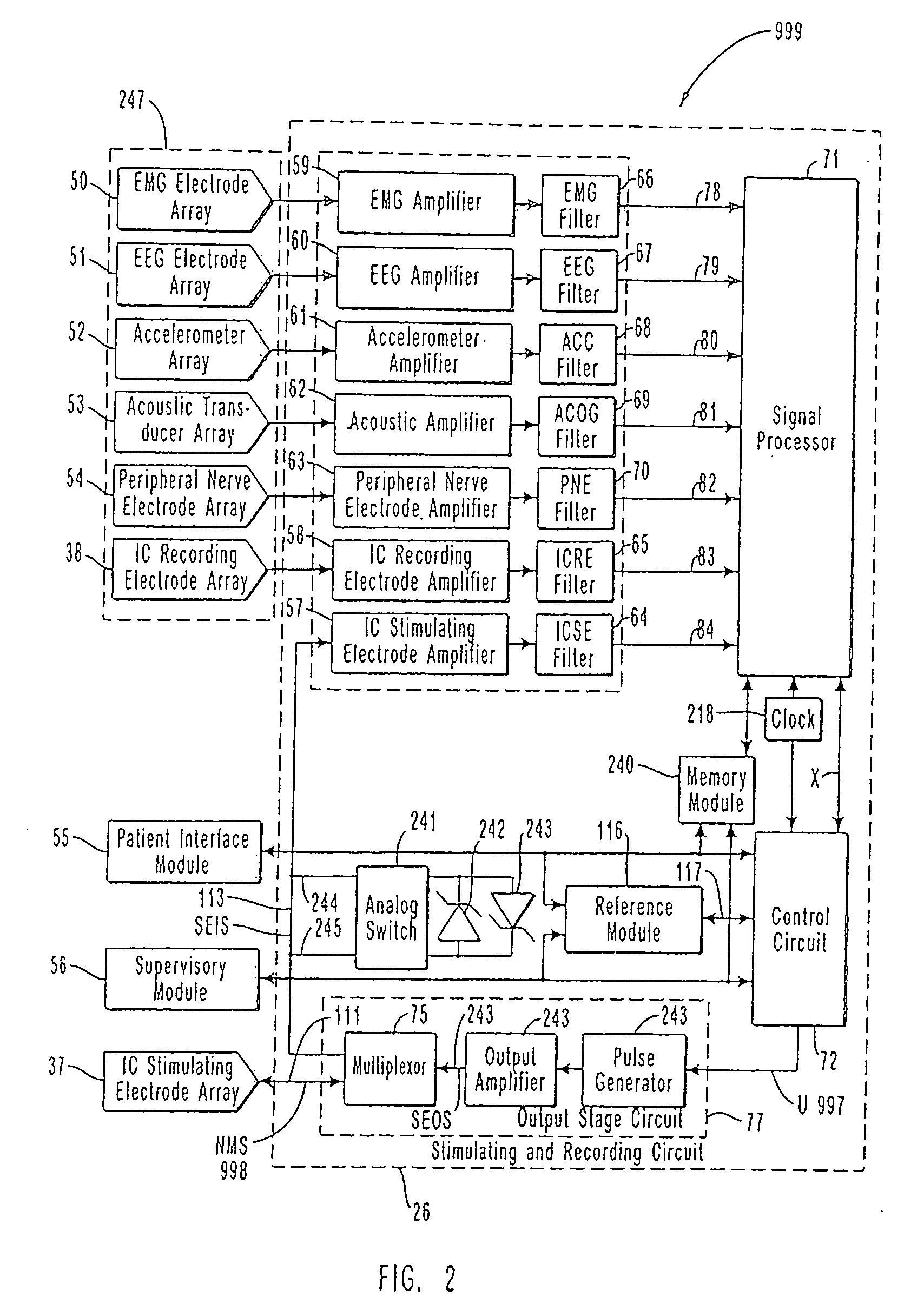
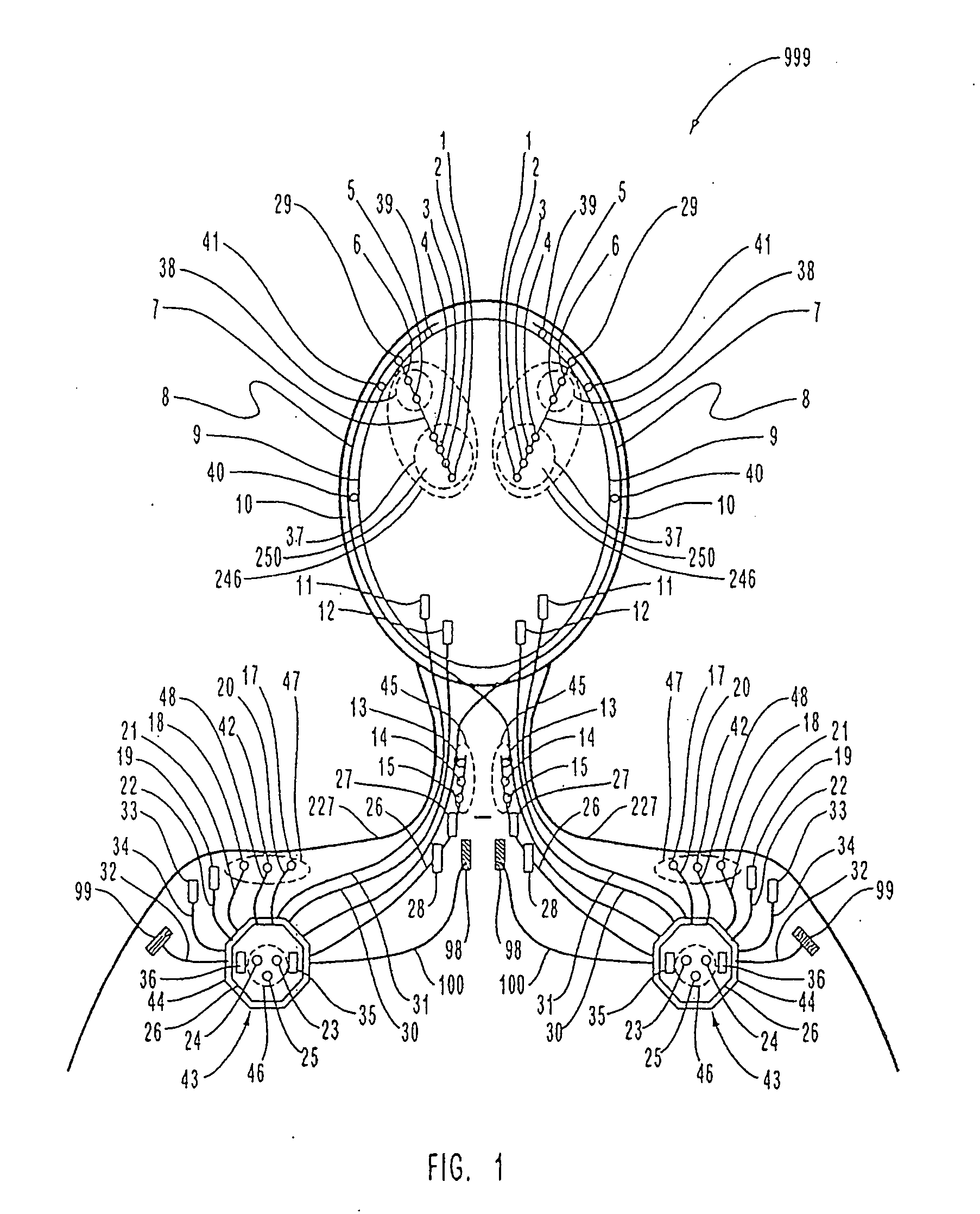
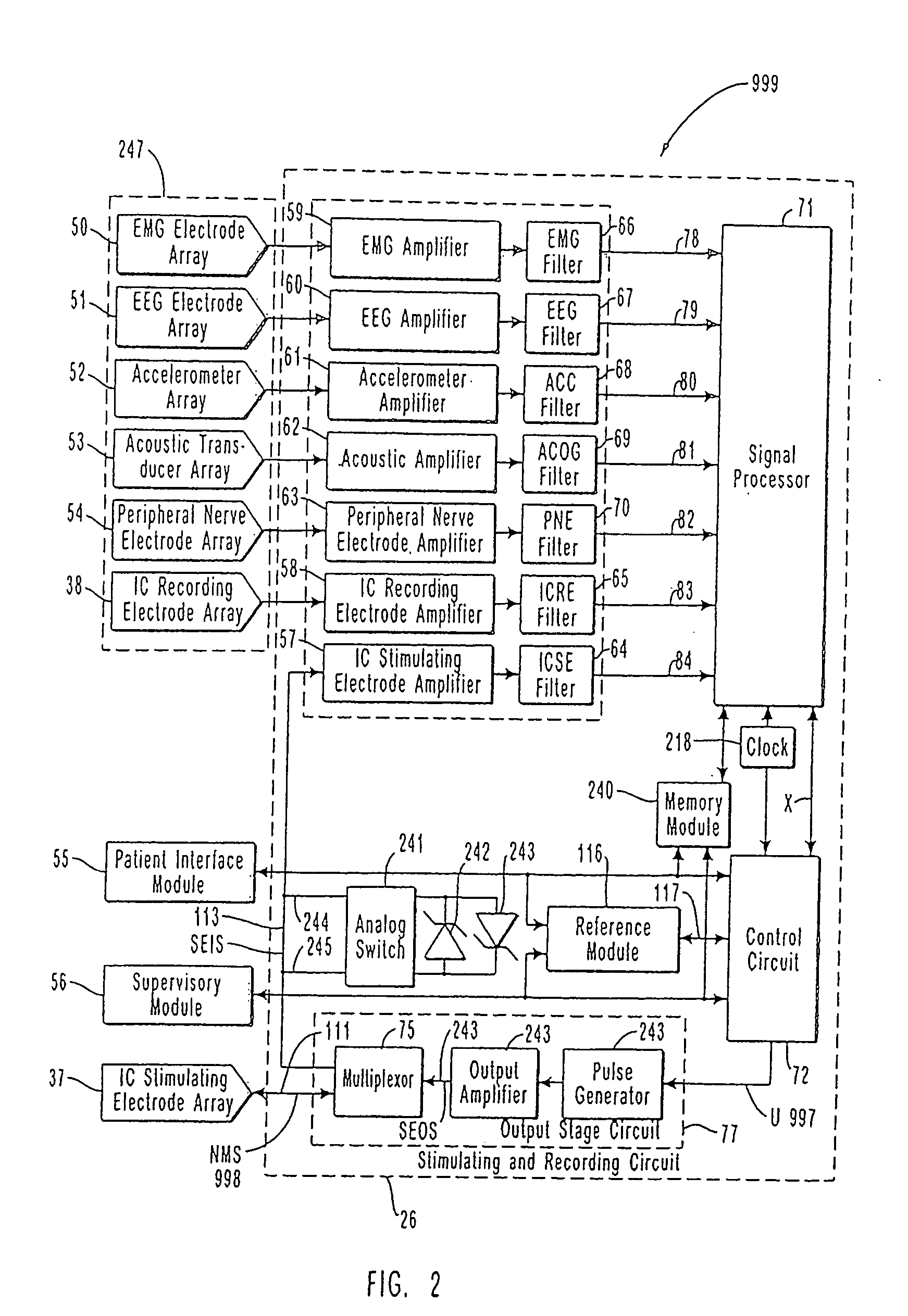
InactiveUS7231254B2Reducing time and number of interactionSatisfactory treatmentHead electrodesImplantable neurostimulatorsNeurological signNervous system
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators to control neurological state and prevent neurological signs and symptoms. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Closed-loop feedback-driven neuromodulation
InactiveUS20050240242A1Shorten the timeSatisfactory treatmentHead electrodesImplantable neurostimulatorsNeurological signNervous system
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators to control neurological state and prevent neurological signs and symptoms. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Monitoring efficacy of neural modulation therapy
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators to control neurological state and prevent neurological signs and symptoms. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Methods and systems for continuous EEG monitoring
InactiveUS20070161919A1Enhanced signalElectroencephalographyHead electrodesNervous systemNeurological signs
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators to control neurological state and prevent neurological signs and symptoms. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Treatment of renal hypertension or carotid sinus syndrome with adventitial pharmaceutical sympathetic denervation or neuromodulation
ActiveUS20110104061A1Improve concentrationOrganic active ingredientsBacterial antigen ingredientsRenal HypertensionsCvd risk
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict medication regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST
Methods and systems for determining subject-specific parameters for a neuromodulation therapy
A neurological control system for modulating activity of any component or structure comprising the entirety or portion of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” The neurological control system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators to control neurological state and prevent neurological signs and symptoms. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals sensed by one or more sensors, each configured to sense a particular characteristic indicative of a neurological or psychiatric condition.
Owner:CYBERONICS INC
Clostridial toxin derivatives able to modify peripheral sensory afferent functions
InactiveUS6395513B1Pain reliefReduce and preferably prevent transmissionNervous disorderPeptide/protein ingredientsClostridial toxinProjection neuron
The invention relates to an agent specific for peripheral sensory afferents. The agent may inhibit the transmission of signals between a primary sensory afferent and a projection neuron by controlling the release of at least one neurotransmitter or neuromodulator from the primary sensory afferent. The agent may be used in or as a pharmaceutical for the treatment of pain, particularly chronic pain.
Owner:HEALTH PROTECTION AGENCY +1
Treatment of hypertension by renal vascular delivery of guanethidine
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or sympathetic nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST
Closed-loop autonomic neuromodulation for optimal control of neurological and metabolic disease
InactiveUS7974696B1Shorten the timeSatisfactory treatmentElectroencephalographyHead electrodesDiseaseNervous system
A neurological control system for modulating activity of any component or structure of some or all of the nervous system, or any structure interfaced thereto, generally referred to herein as a “nervous system component.” This system generates neural modulation signals delivered to a nervous system component through one or more neuromodulators to control neurological state or autonomic state and prevent neurological or metabolic signs and symptoms. Such treatment parameters may be derived from a neural response to previously delivered neural modulation signals, with sensors configured to sense a particular characteristic indicative of a neurological, psychiatric, or metabolic condition.
Owner:DILORENZO BIOMEDICAL
Controlled release delivery system for nasal application of neurotransmitters
InactiveUS20090227550A1Improve bioavailabilityEffective serum levelOrganic active ingredientsBiocideNoseProgesterones
This invention relates to a galenical gel formulation for nasal administration of neurotransmitters / neuromodulators such as dopamine, serotonin or pregnenolone and progesterone. The special lipophilic or partly lipophilic system of the invention leads to high bioavailability of the active ingredient in plasma and brain caused by sustained serum levels and / or direct or partly direct transport from nose to the brain.
Owner:MATTERN PHARMA
Treatment of hypertension by renal vascular delivery of guanethidine
ActiveUS8399443B2Improve concentrationBiocidePharmaceutical delivery mechanismRegimenRenal Hypertensions
Sympathetic nerves run through the adventitia surrounding renal arteries and are critical in the modulation of systemic hypertension. Hyperactivity of these nerves can cause renal hypertension, a disease prevalent in 30-40% of the adult population. Hypertension can be treated with neuromodulating agents (such as angiotensin converting enzyme inhibitors, angiotensin II inhibitors, or aldosterone receptor blockers), but requires adherence to strict regimens and often does not reach target blood pressure threshold to reduce risk of major cardiovascular events. A minimally invasive solution is presented here to reduce the activity of the sympathetic nerves surrounding the renal artery by locally delivering neurotoxic or sympathetic nerve-blocking agents into the adventitia. Extended elution of these agents may also be accomplished in order to tailor the therapy to the patient.
Owner:MERCATOR MEDSYST INC
Clostridial toxin derivatives able to modify peripheral sensory afferent functions
InactiveUS20030049264A1Pain reliefReduce and preferably prevent transmissionNervous disorderHydrolasesClostridial toxinMedicine
The invention relates to an agent specific for peripheral sensory afferents. The agent may inhibit the transmission of signals between a primary sensory afferent and a projection neutron by controlling the release of at least one neurotransmitter or neuromodulator from the primary sensory afferent. The agent may be used in or as a pharmaceutical for the treatment of pain, particularly chronic pain.
Owner:HEALTH PROTECTION AGENCY +1
Human IgM antibodies, and diagnostic and therapeutic uses thereof particularly in the central nervous system
InactiveUS7473423B2Promote reproductionPromote safer self-therapiesNervous disorderPeptide/protein ingredientsNervous systemIgm antibody
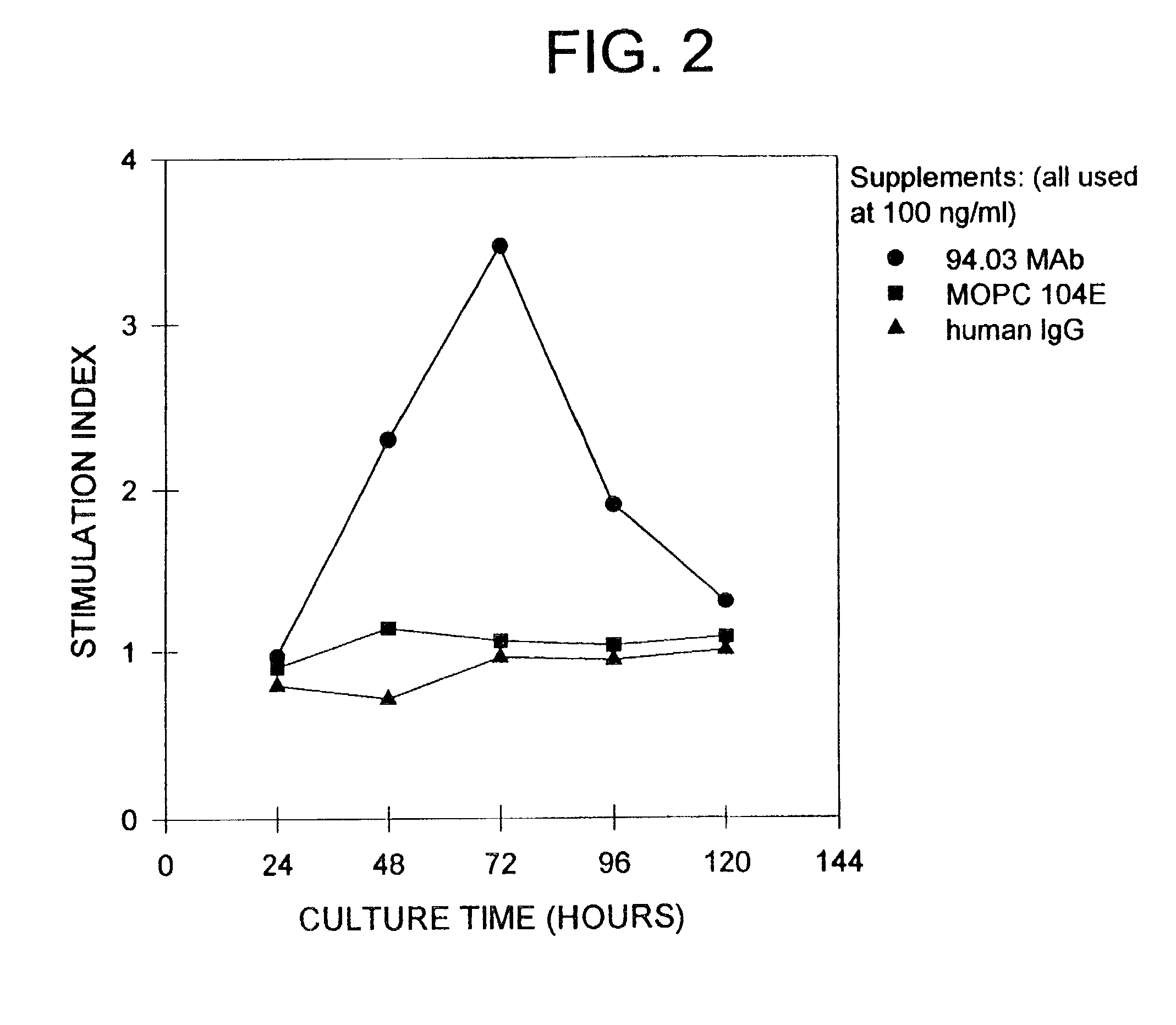
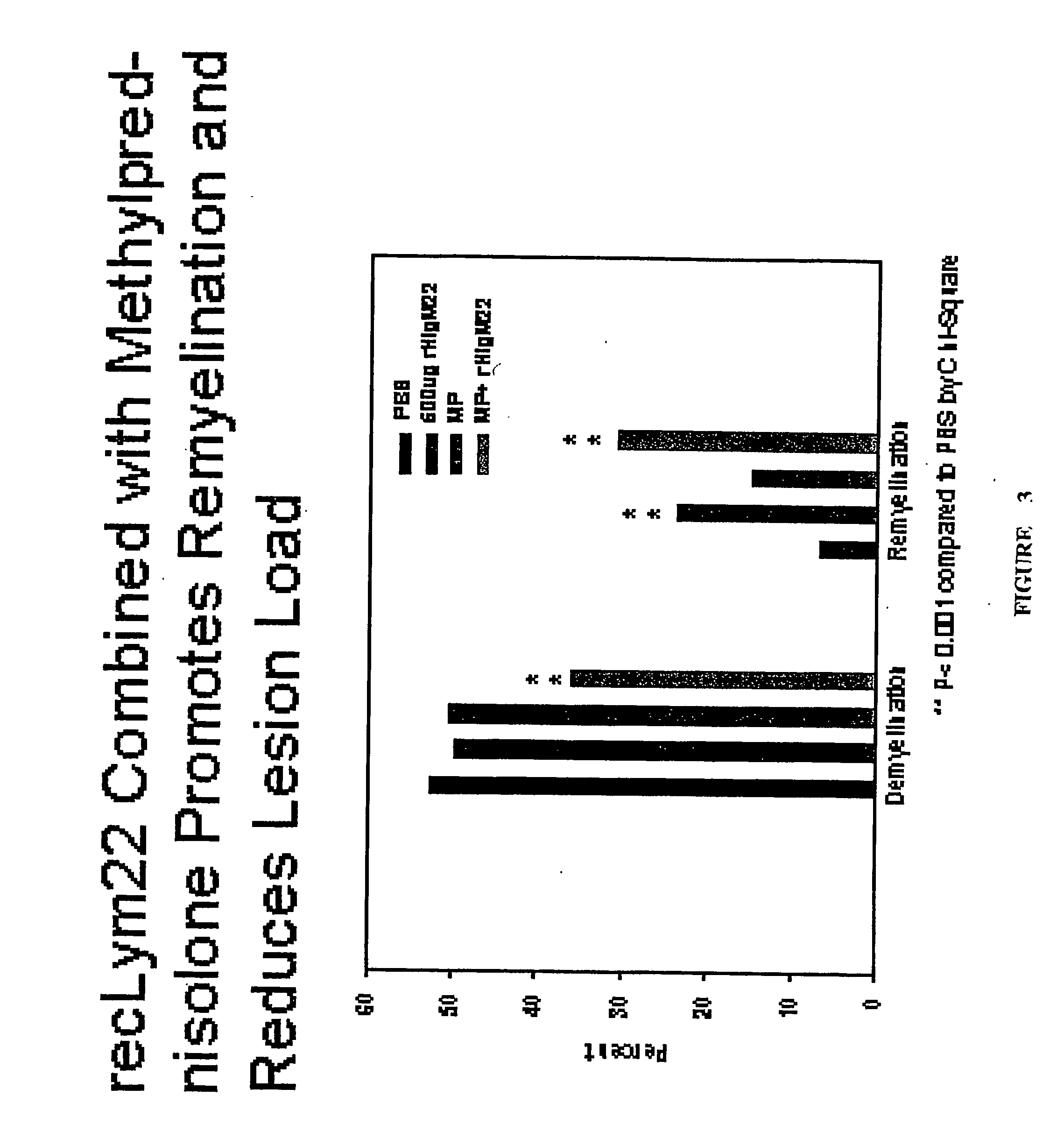
Antibodies, and particularly human antibodies, are disclosed that demonstrate activity in the treatment of demyelinating diseases as well as other diseases of the central nervous system that are of viral, bacterial or idiopathic origin, including neural dysfunction caused by spinal cord injury. Neuromodulatory agents are set forth that include and comprise a material selected from the group consisting of an antibody capable of binding structures or cells in the central nervous system, a peptide analog, a hapten, active fragments thereof, agonists thereof, mimics thereof, monomers thereof and combinations thereof. The neuromodulatory agent has one or more of the following characteristics: it is capable of inducing remyelination; binding to neural tissue; promoting Ca++ signaling with oligodendrocytes; and promoting cellular proliferation of glial cells. Amino acid and DNA sequences of exemplary antibodies are disclosed. Methods are described for treating demyelinating diseases, and diseases of the central nervous system of humans and domestic animals, using polyclonal IgM antibodies and human monoclonal antibodies sHIgm22(LYM 22), sHIgm46(LYM46) ebvHIgM MSI19D10, CB2bG8, AKJR4, CB2iE12, CB2iE7, MSI19E5 and MSI10E10, active fragments thereof and the like. The invention also extends to the use of human antibodies, fragments, peptide derivatives and like materials, and their use in diagnostic and therapeutic applications, including screening assays for the discovery of additional antibodies that bind to cells of the nervous system, particularly oligodendrocytes.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
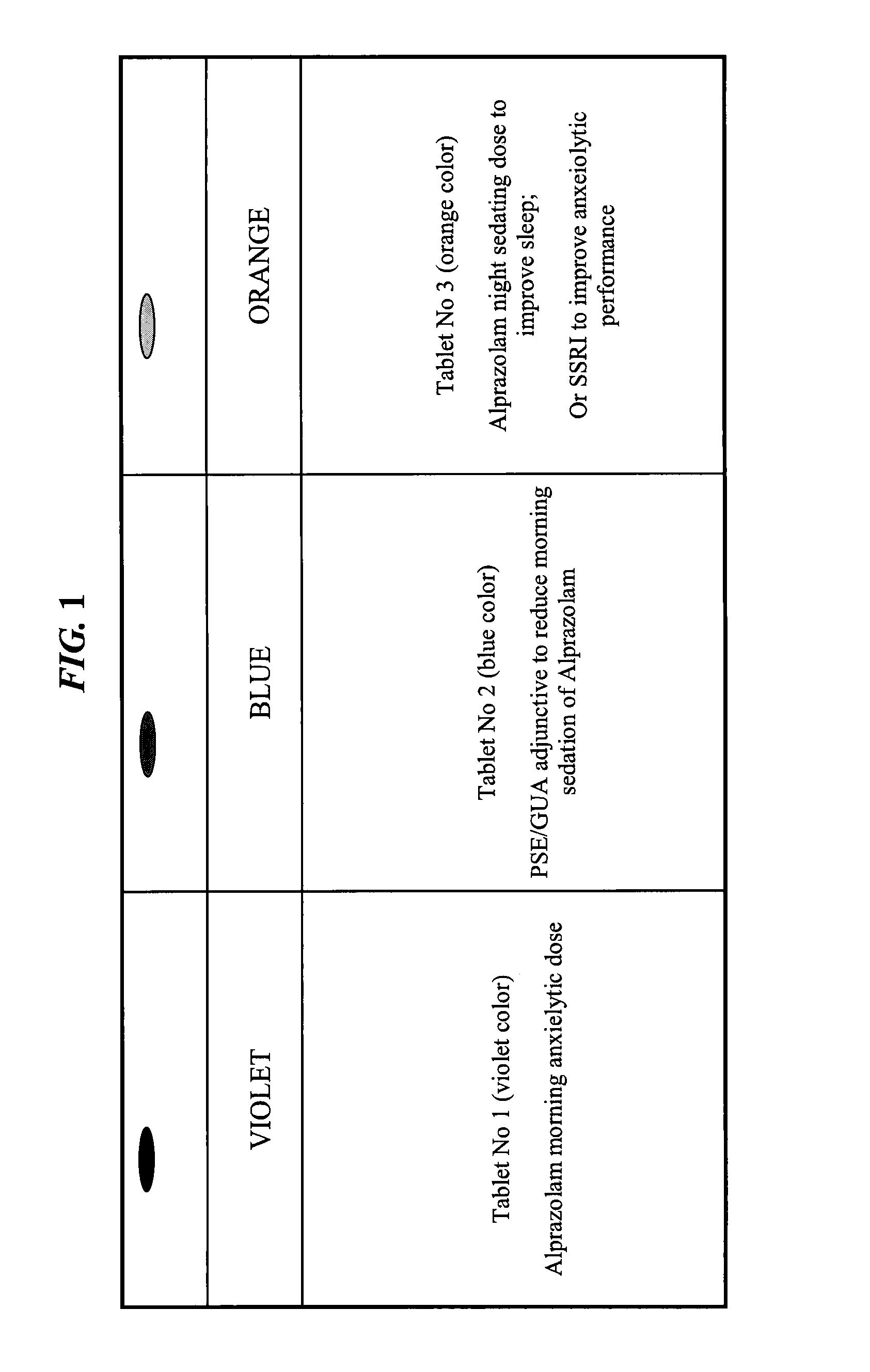
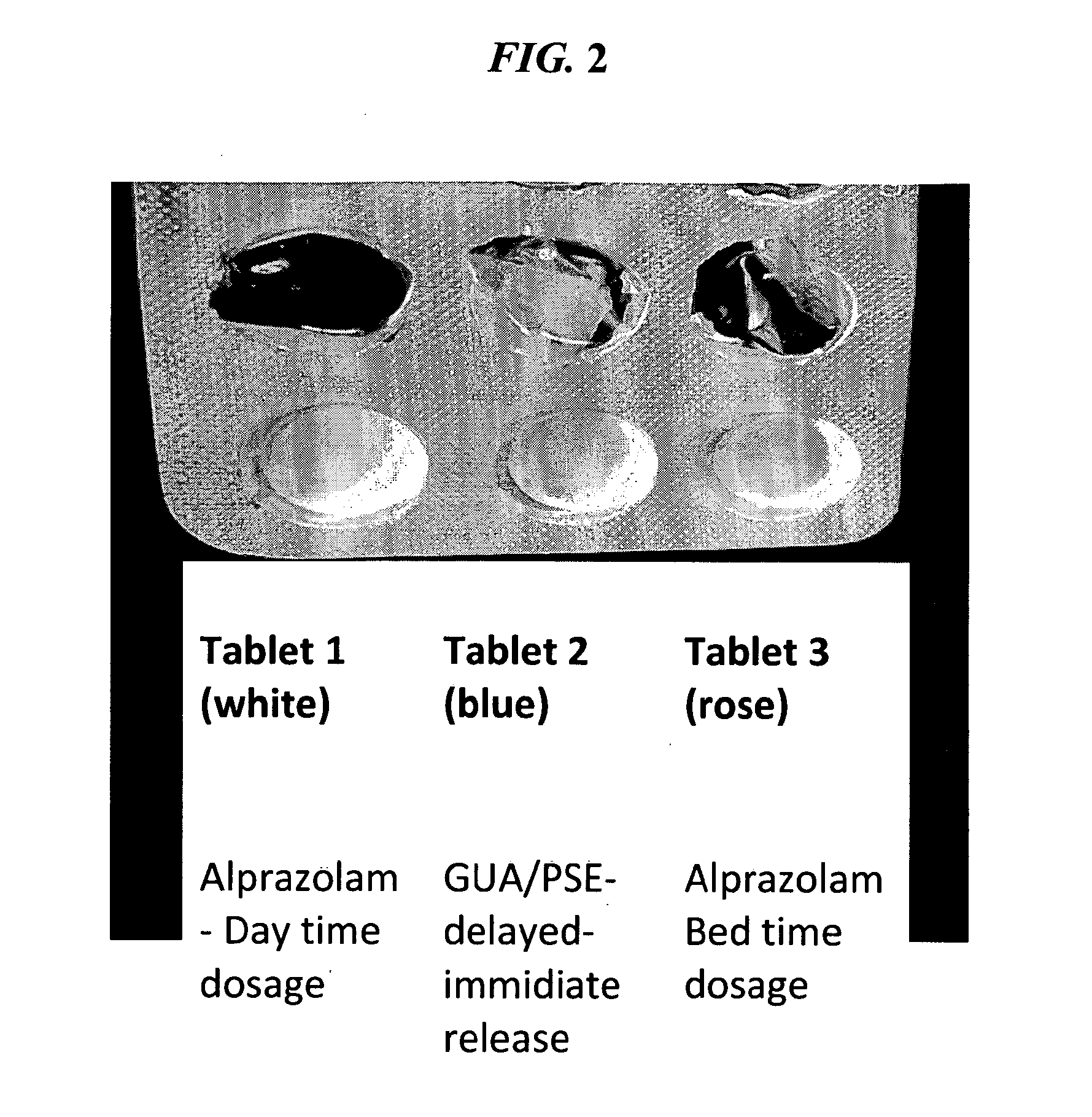
CNS pharmaceutical compositions and methods of use
InactiveUS20110054038A1Eliminate side effectsReduce dependenceBiocideNervous disorderOral medicationSide effect
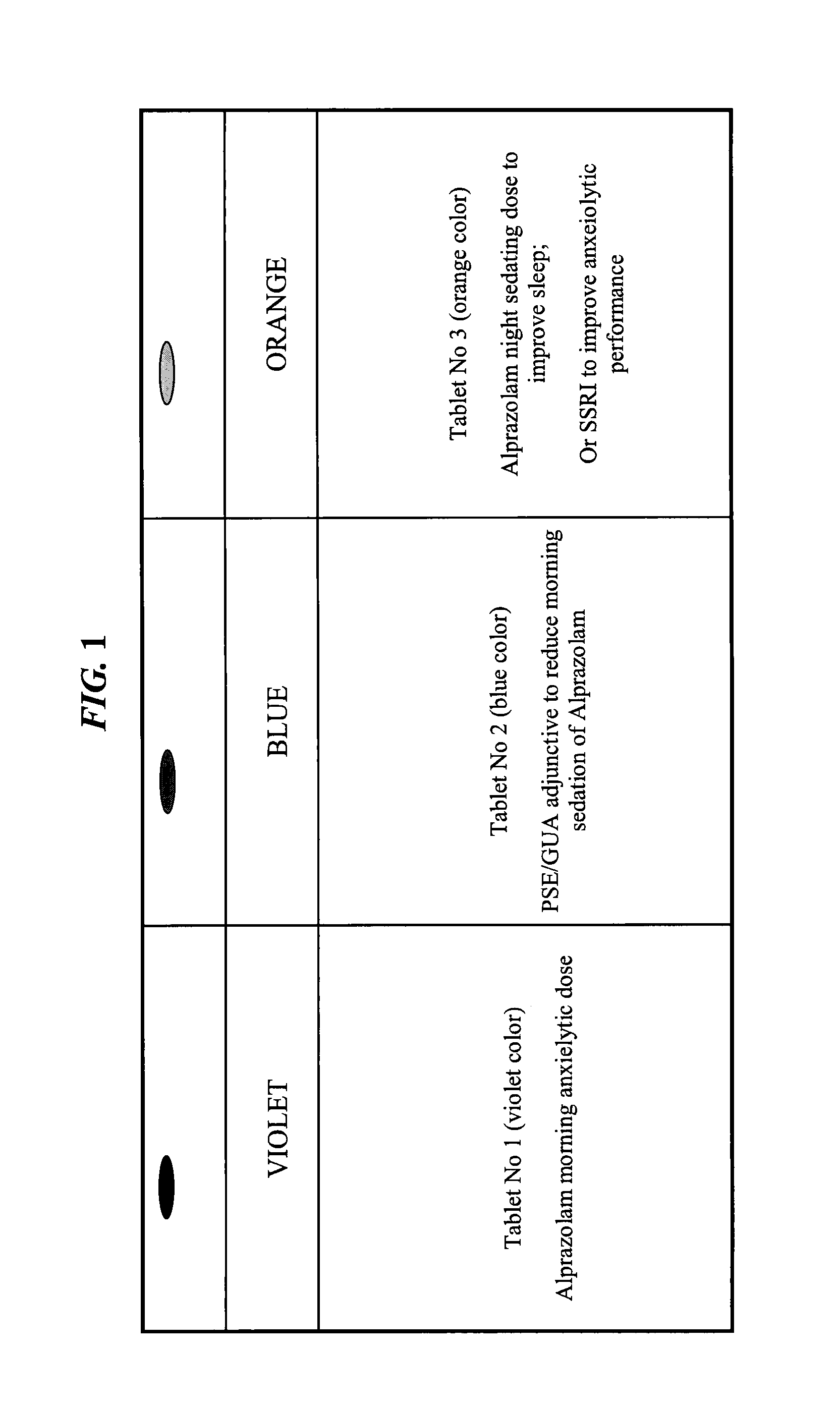
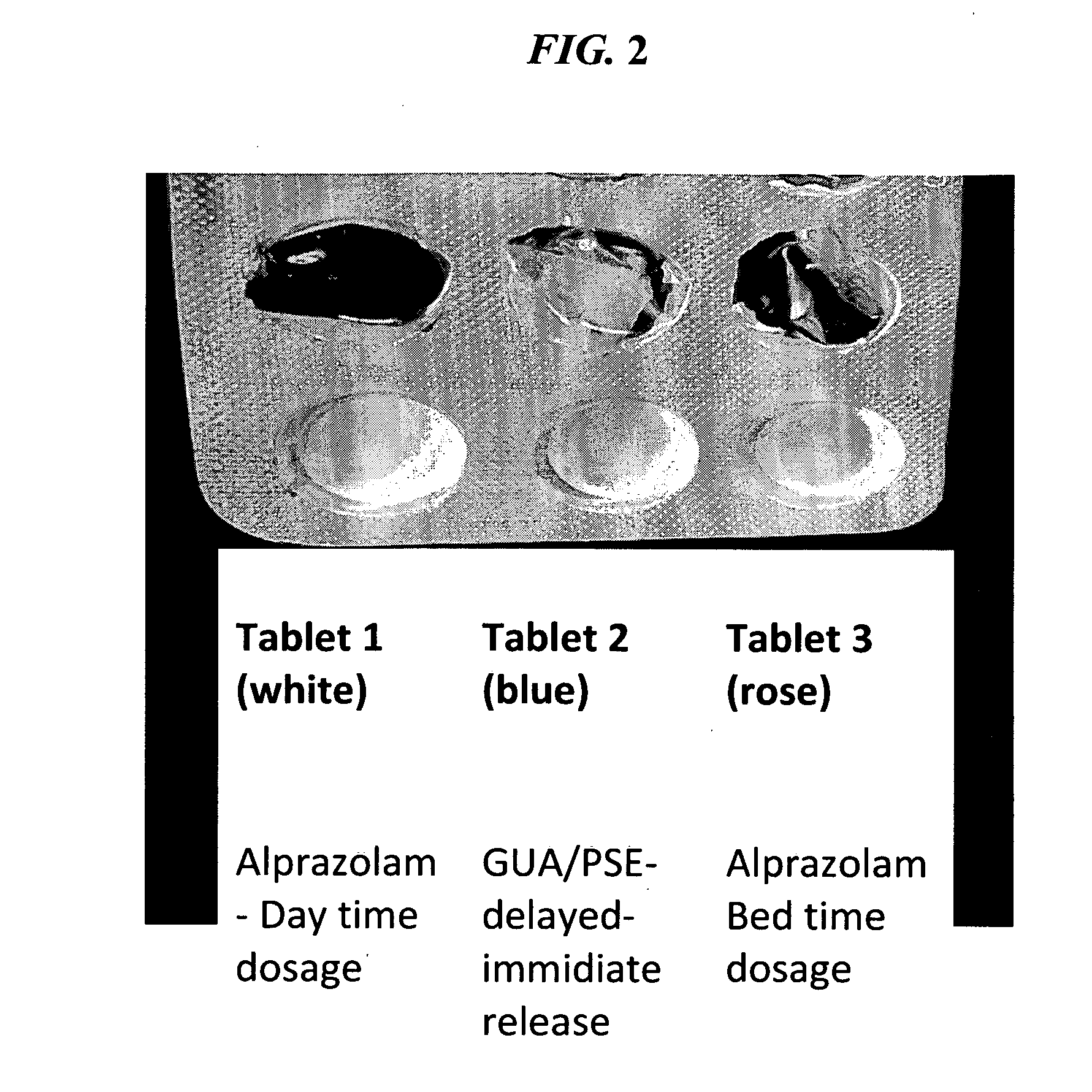
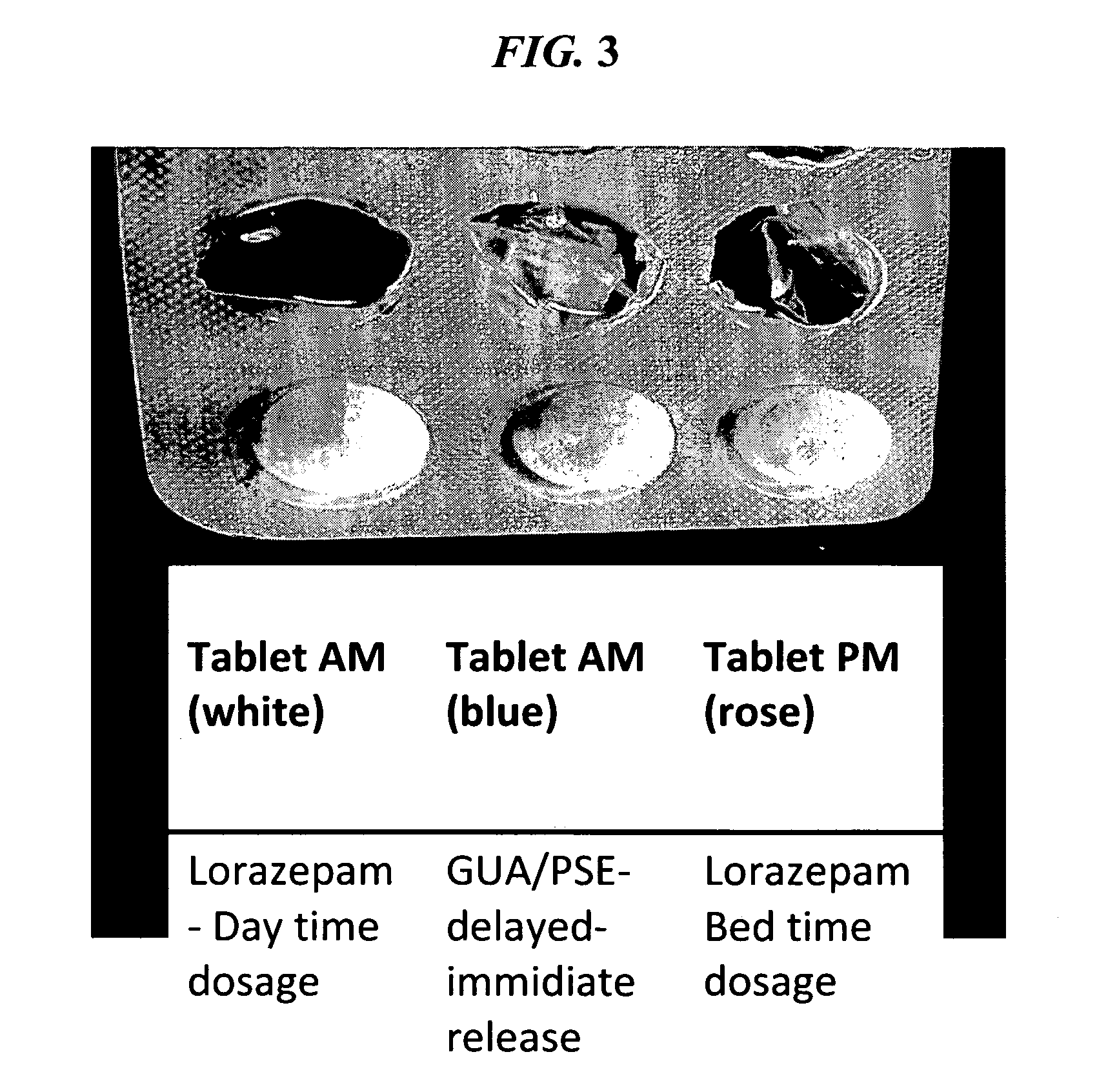
The present invention is directed to CNS pharmaceutical compositions and methods of use. The pharmaceutical compositions comprise a CNS active agent and preferably at least two vagal neuromodulators, one of which is a mechanoreceptor stimulator. The vagal neuromodulators are preferably in an amount sufficient to reduce a somnolence side-effect of the CNS active agent without changing its therapeutic efficacy / activity. The invention further encompasses a method of reducing CNS active agent side-effects. The method typically comprises oral administration of at least one CNS active agent to a patient at the conventionally accepted dose; and administration of at least two vagal neuromodulators to the patient so that at least one neuromodulator is administered or released from dosage form after the CNS active agent is administered and / or released.
Owner:TARGIA PHARMA
Neuromodulators and Methods of Use
ActiveUS20110224145A1Restore visionAdd dimensionOrganic active ingredientsSenses disorderRetinaNeuron
The present disclosure provides neuromodulators, nucleic acid encoding thereof, and compositions thereof for endowing visual processing abilities to neuronal cells. The present disclosure further provides a method of restoring light sensitivity to degenerate retinas.
Owner:RGT UNIV OF CALIFORNIA
Controlled release delivery system for nasal application of neurotransmitters
ActiveUS20120009249A1Improve bioavailabilityEffective levelingBiocideOrganic active ingredientsNoseProgesterones
This invention relates to a galenical gel formulation for nasal administration of neurotransmitters / neuromodulators such as dopamine, serotonin or pregnenolone and progesterone. The special lipophilic or partly lipophilic system of the invention leads to high bioavailability of the active ingredient in plasma and brain caused by sustained serum levels and / or direct or partly direct transport from nose to the brain.
Owner:M & P PHARMA
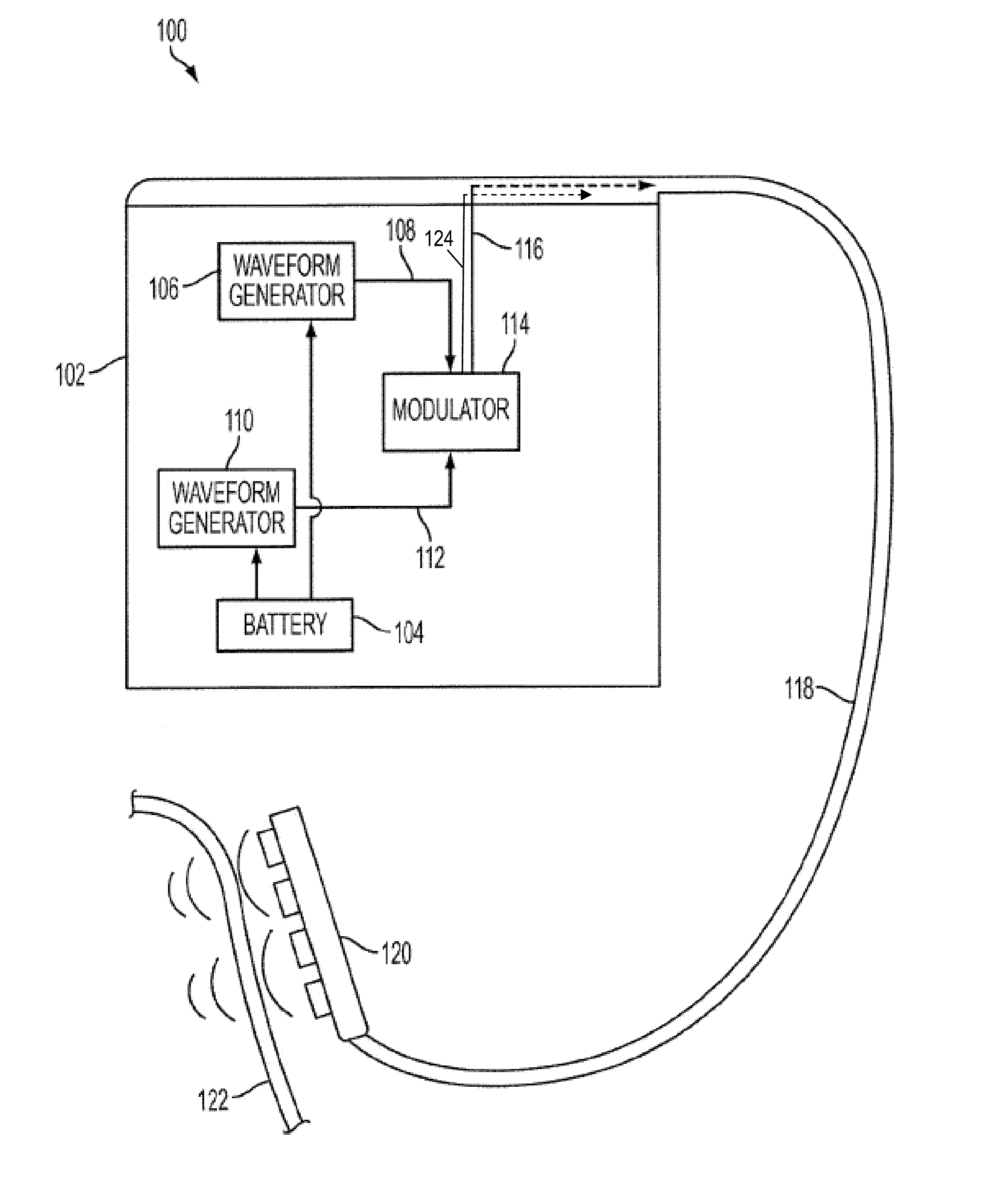
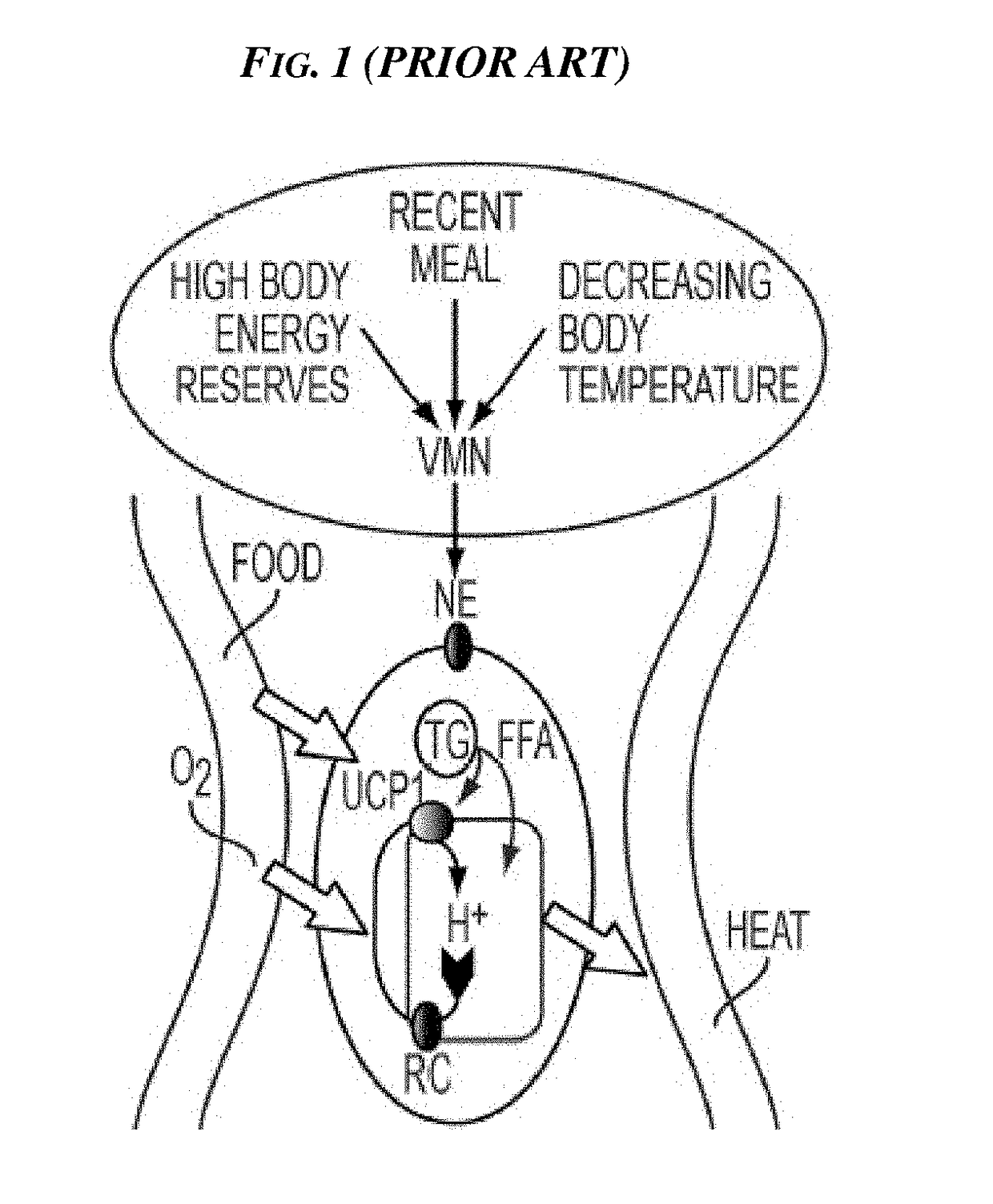
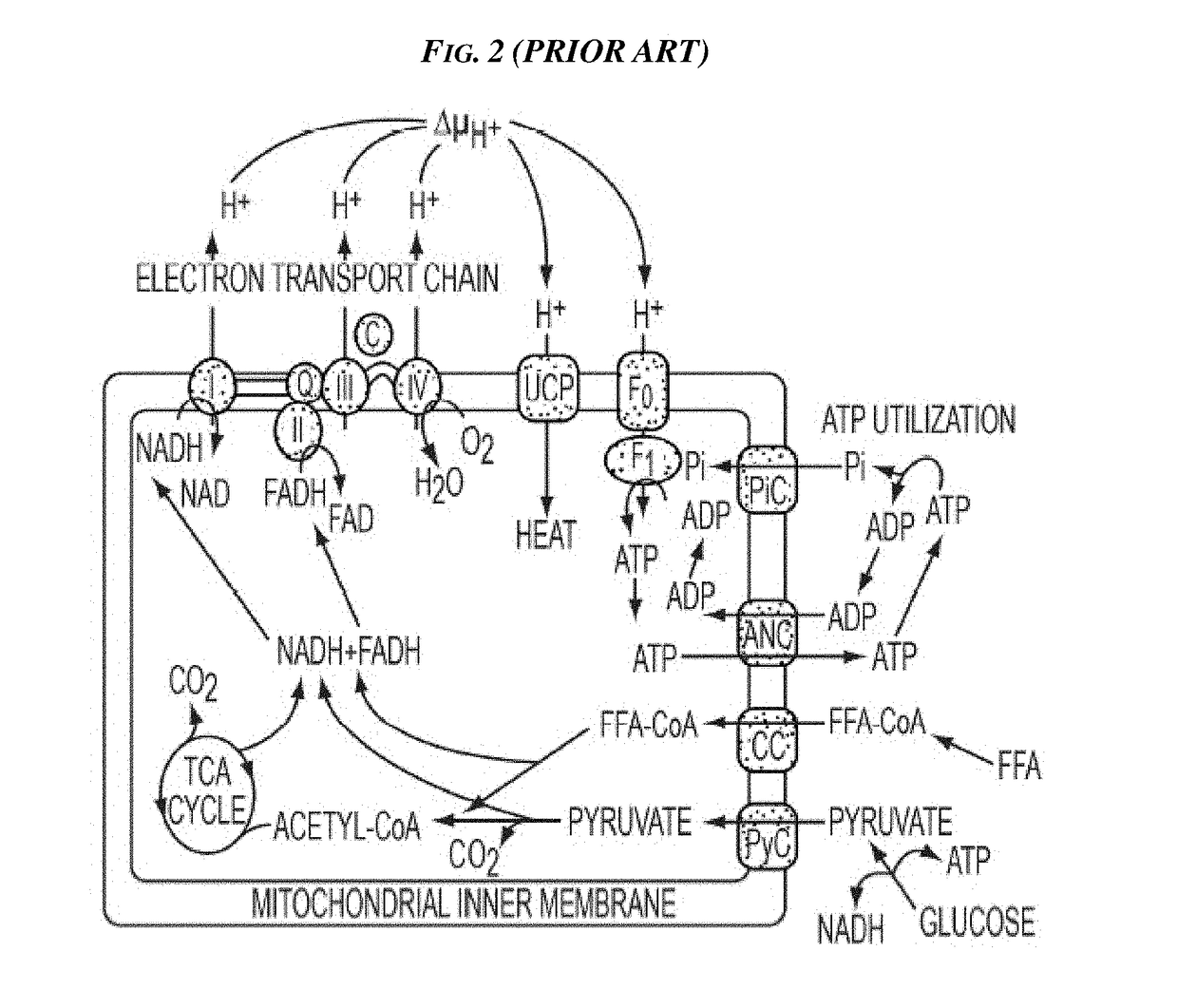
Methods and Devices for Inhibiting Nerves When Activating Brown Adipose Tissue
ActiveUS20160184568A1Increased energy expenditureMedical devicesMedical applicatorsMicrochiropteraBrown adipose tissue
Methods and devices are provided for inhibiting nerves when activating brown adipose tissue (BAT). In general, a first nerve type (e.g., sympathetic nerves) innervating BAT can be activated while at least one other nerve type (e.g., parasympathetic nerves and / or sensory nerves) innervating BAT is being suppressed. A first neuromodulator (e.g., an electrical signal, a chemical, a light, cooling, etc.) can be applied to activate the first nerve type, and a second neuromodulator can be applied to inhibit the at least one other nerve type. In this way, parasympathetic nerves and / or sensory nerves innervating BAT can be inhibited when activating sympathetic nerves innervating BAT.
Owner:CILAG GMBH INT
Substituted 6−membered n−heterocyclic compounds and their uses as neurological regulator
InactiveUS20050130958A1Promoting outgrowthPromote regenerationNervous disorderOrganic chemistryProteinase activityProtease
This invention relates to substituted 6-membered N-Herterocyclic neurotrophic compounds of formula (I) or pharmaceutically acceptable salts or hydrates thereof, wherein R1, R2, X, Y, and Z are as defined in the description; their preparation methods, compositions comprising the same, and their use as inhibitors of FK560 binding proteases activity for treating and preventing neurodegenerative diseases and other nerve disorders associated with nerve injuries or other related diseases.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Neuromodulating compositions and related therapeutic methods for the treatment of cancer by modulating an Anti-cancer immune response
InactiveUS20190240293A1Relieve symptomsLimited ability to cross blood brain barrierNervous disorderPeptide/protein ingredientsCancer researchNeuroregulator
Described herein are methods for treating a subject having or at risk of developing cancer administering a neuromodulating agent.
Owner:FLAGSHIP PIONEERING INNOVATIONS V INC
Human IgM antibodies, and diagnostic and therapeutic uses thereof particularly in the central nervous system
InactiveUS20090274690A1Promote safer self-therapiesExtended half-lifeNervous disorderPeptide/protein ingredientsNervous systemSpinal cord lesion
Antibodies, and particularly human antibodies, are disclosed that demonstrate activity in the treatment of demyelinating diseases as well as other diseases of the central nervous system that are of viral, bacterial or idiopathic origin, including neural dysfunction caused by spinal cord injury. Neuromodulatory agents are set forth that include and comprise a material selected from the group consisting of an antibody capable of binding structures or cells in the central nervous system, a peptide analog, a hapten, active fragments thereof, agonists thereof, mimics thereof, monomers thereof and combinations thereof. The neuromodulatory agent has one or more of the following characteristics: it is capable of inducing remyelination; binding to neural tissue; promoting Ca−− signaling with oligodendrocytes; and promoting cellular proliferation of glial cells. Amino acid and DNA sequences of exemplary antibodies are disclosed. Methods are described for treating demyelinating diseases, and diseases of the central nervous system of humans and domestic animals, using polyclonal IgM antibodies and human monoclonal antibodies sHIgm22(LYM 22), sHIgm46(LYM46) ebvHIgM MSI19D10, CB2bG8, AKJR4, CB2iE12, CB2iE7, MSI19E5 and MSI10E10, active fragments thereof and the like. The invention also extends to the use of human antibodies, fragments, peptide derivatives and like materials, and their use in diagnostic and therapeutic applications, including screening assays for the discovery of additional antibodies that bind to cells of the nervous system, particularly oligodendrocytes.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Receptor regulator
InactiveUS20060252797A1Good natureSafe and usefulBiocideNervous disorderNeuromedin U receptorNeuroregulator
A compound having a partial structure represented by the formula (A) (wherein ring Xa represents a nitrogen-containing ring and R represents optionally substituted amino) or a salt thereof. The compound or salt is highly effective in regulating neuromedin U receptors and is useful as a preventive / therapeutic agent for hypertension, etc.
Owner:TAKEDA PHARMA CO LTD
Methods and devices for inhibiting nerves when activating brown adipose tissue
ActiveUS10092738B2Increased energy expenditureMedical devicesMedical applicatorsMicrochiropteraBrown adipose tissue
Methods and devices are provided for inhibiting nerves when activating brown adipose tissue (BAT). In general, a first nerve type (e.g., sympathetic nerves) innervating BAT can be activated while at least one other nerve type (e.g., parasympathetic nerves and / or sensory nerves) innervating BAT is being suppressed. A first neuromodulator (e.g., an electrical signal, a chemical, a light, cooling, etc.) can be applied to activate the first nerve type, and a second neuromodulator can be applied to inhibit the at least one other nerve type. In this way, parasympathetic nerves and / or sensory nerves innervating BAT can be inhibited when activating sympathetic nerves innervating BAT.
Owner:CILAG GMBH INT
CNS pharmaceutical compositions and methods of use
ActiveUS20140256709A1Eliminate side effectsReduce dependenceBiocideNervous disorderSide effectOral medication
The present invention is directed to CNS pharmaceutical compositions and methods of use. The pharmaceutical compositions comprise a CNS active agent and preferably at least two vagal neuromodulators, one of which is a mechanoreceptor stimulator. The vagal neuromodulators are preferably in an amount sufficient to reduce a somnolence side-effect of the CNS active agent without changing its therapeutic efficacy / activity. The invention further encompasses a method of reducing CNS active agent side-effects. The method typically comprises oral administration of at least one CNS active agent to a patient at the conventionally accepted dose; and administration of at least two vagal neuromodulators to the patient so that at least one neuromodulator is administered or released from dosage form after the CNS active agent is administered and / or released.
Owner:TARGIA PHARMA
CNS pharmaceutical compositions and methods of use
InactiveUS8729070B2Eliminate side effectsReduce dependenceBiocideNervous disorderOral medicationSide effect
Owner:TARGIA PHARMA
Methods of inducing photosensitivity by targeting channelrhodopsin-2 and halorhodopsin to subcellular regions of retinal ganglion cells
ActiveUS9545422B2AbilityRestore visionOrganic active ingredientsSenses disorderNeuronVisual perception
The present disclosure provides neuromodulators, nucleic acid encoding thereof, and compositions thereof for endowing visual processing abilities to neuronal cells. The present disclosure further provides a method of restoring light sensitivity to degenerate retinas.
Owner:RGT UNIV OF CALIFORNIA
Method and a system for creating dynamic neural function libraries
ActiveUS20160019457A1Increased complexityReduce intensityDigital computer detailsNeural architecturesSynapseDynamic neural network
A method for creating a dynamic neural function library that relates to Artificial Intelligence systems and devices is provided. Within a dynamic neural network (artificial intelligent device), a plurality of control values are autonomously generated during a learning process and thus stored in synaptic registers of the artificial intelligent device that represent a training model of a task or a function learned by the artificial intelligent device. Control Values include, but are not limited to, values that indicate the neurotransmitter level that is present in the synapse, the neurotransmitter type, the connectome, the neuromodulator sensitivity, and other synaptic, dendric delay and axonal delay parameters. These values form collectively a training model. Training models are stored in the dynamic neural function library of the artificial intelligent device. The artificial intelligent device copies the function library to an electronic data processing device memory that is reusable to train another artificial intelligent device.
Owner:BRAINCHIP INC
Substituted 6-membered N-heterocyclic compounds and method for their use as neurological regulator
This invention relates to substituted 6-membered N-Herterocyclic neurotrophic compounds of formula (I) or pharmaceutically acceptable salts or hydrates thereof, wherein R1, R2, X, Y, and Z are as defined in the description; their preparation methods, compositions comprising the same, and their use as inhibitors of FK560 binding proteases activity for treating and preventing neurodegenerative diseases and other nerve disorders associated with nerve injuries or other related diseases
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Method and A System for Creating Dynamic Neural Function Libraries
ActiveUS20190012597A1Increased complexityReduce intensityNeural architecturesPhysical realisationSynapseDynamic neural network
A method for creating a dynamic neural function library that relates to Artificial Intelligence systems and devices is provided. Within a dynamic neural network (artificial intelligent device), a plurality of control values are autonomously generated during a learning process and thus stored in synaptic registers of the artificial intelligent device that represent a training model of a task or a function learned by the artificial intelligent device. Control Values include, but are not limited to, values that indicate the neurotransmitter level that is present in the synapse, the neurotransmitter type, the connectome, the neuromodulator sensitivity, and other synaptic, dendric delay and axonal delay parameters. These values form collectively a training model. Training models are stored in the dynamic neural function library of the artificial intelligent device. The artificial intelligent device copies the function library to an electronic data processing device memory that is reusable to train another artificial intelligent device.
Owner:BRAINCHIP INC
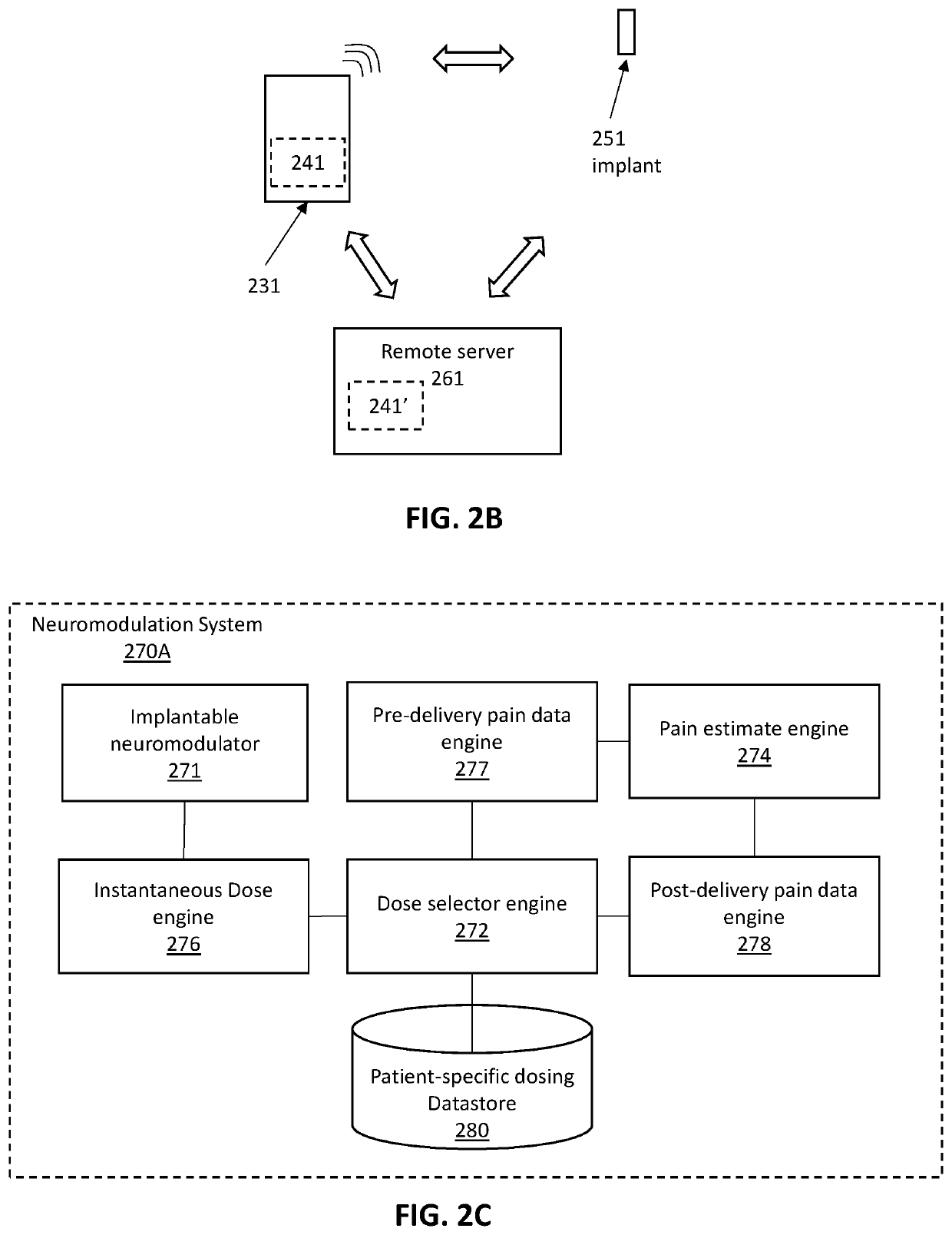
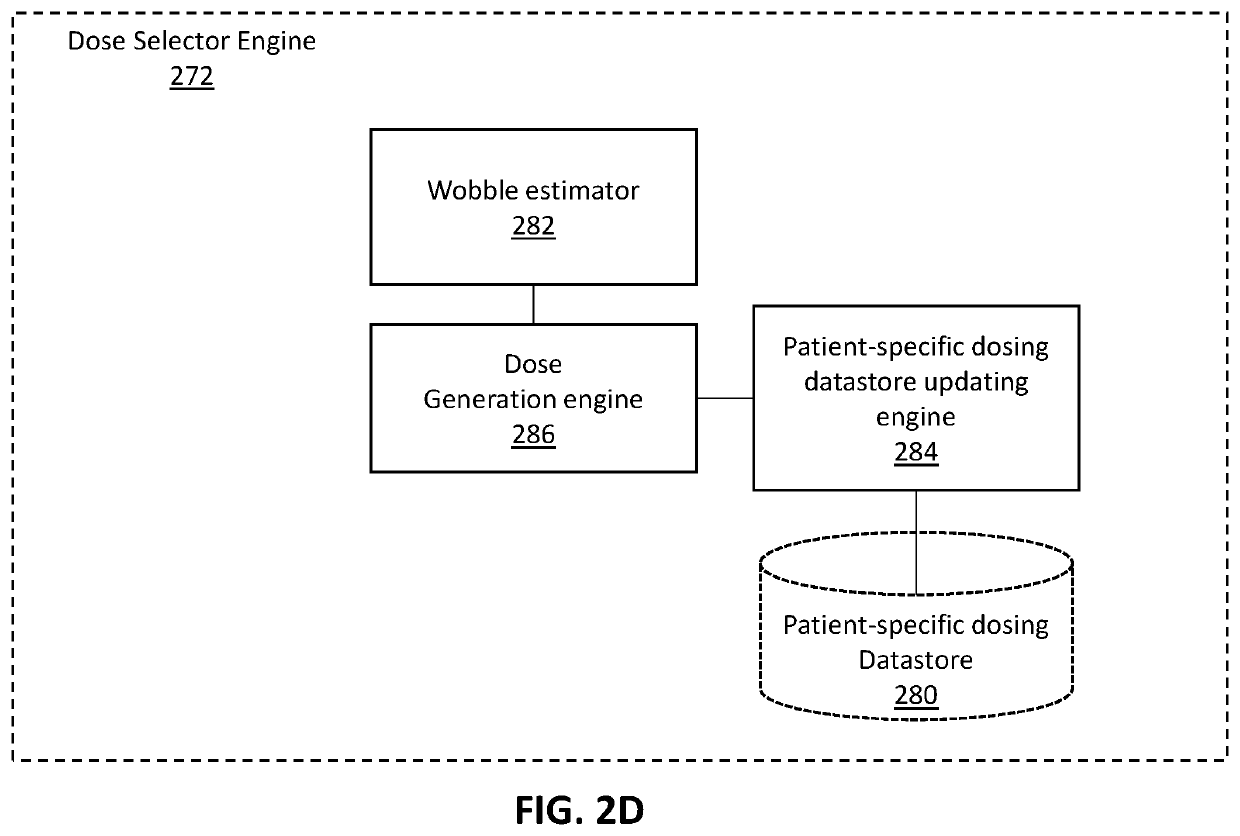
Apparatuses and methods for adjusting a therapeutic electrical dose
PendingUS20210220642A1Spinal electrodesDiagnostic recording/measuringIntensive care medicineBiomedical engineering
Apparatuses and methods of using them for setting a therapeutic dose of a neuromodulator implanted into a patient are described. The methods and apparatuses described herein may include determining the dose based on a patient-specific database of previously delivered dose parameters and corresponding pre-delivery and post-delivery pain estimates from the patient.
Owner:NEUROS MEDICAL
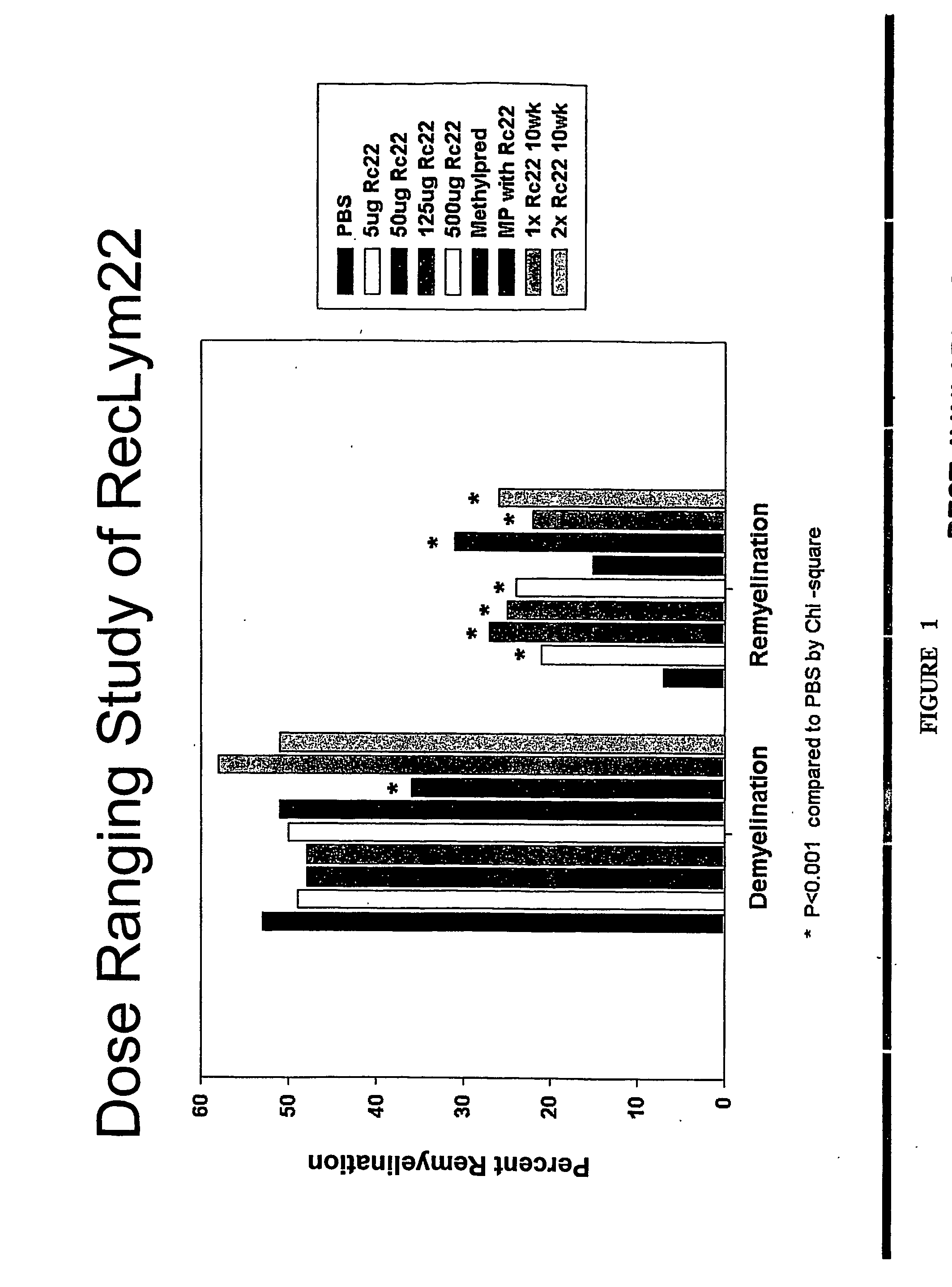
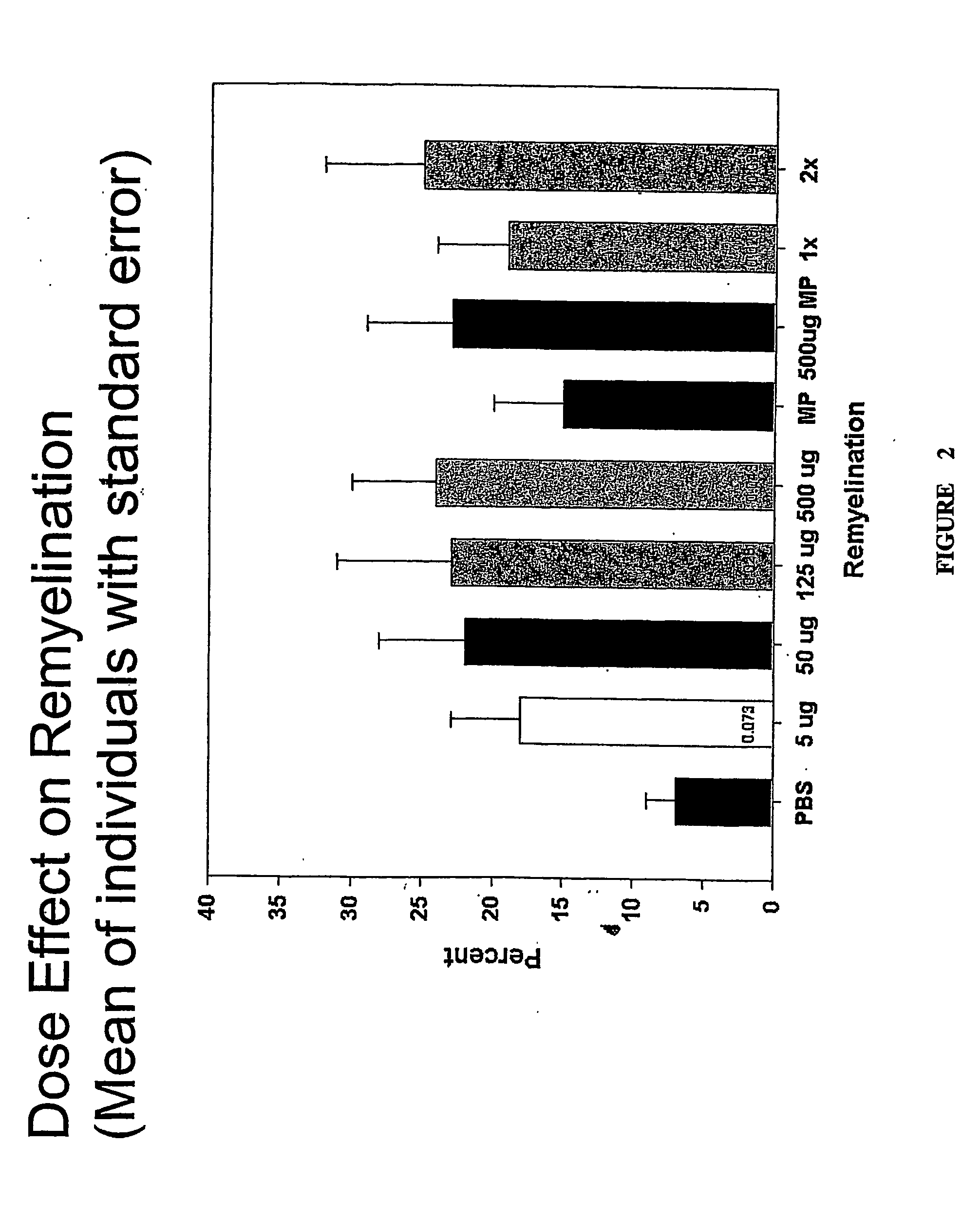
Compositions and methods including a recombinant human mab that promotes cns remyelination
ActiveUS20070086999A1Promoting remyelinationPreventing demyelinationOrganic active ingredientsBiocideNervous systemIgm antibody
Antibodies, and particularly human antibodies, are disclosed that demonstrate activity in the treatment of demyelinating diseases as well as other diseases of the central nervous system that are of viral, bacterial or idiopathic origin, including dysfunction caused by spinal cord injury. Neuromodulatory agents are set forth that include and comprise a material selected from the group consisting of an antibody capable of binding structures or cells in the central nervous system, a peptide analog, a hapten, active fragments thereof, agonists thereof, mimics thereof, monomers thereof and combinations thereof. Methods are described for treating demyelinating diseases, and diseases of the central nervous system of humans and domestic animals, using polyclonal IgM antibodies and human monoclonal antibodies sHIgm22(LYM 22), sHIgm46(LYM46) ebvHIgM MSI19D10, CB2bG8, AKJR4, CB2iE12, CB2iE7, MSI19E5 and MSI10E10, active fragments thereof and the like. The invention also extends to the use of human antibodies, fragments, peptide derivatives and like materials, and their use in above referenced therapeutic applications, and to pharmaceutical compositions containing them, that may be administered in desirably low doses to treat conditions involving demyelination and to promote remyelination.
Owner:ACORDA THERAPEUTICS INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com