Method for preparing goserelin slow-release implant
A technology of sustained-release implants and goserelin, which is applied in the pharmaceutical field, can solve the problems of reducing time cost and short solvent removal time, so as to improve production efficiency, shorten drying time, and facilitate removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Primary screening of low boiling point solvents
[0039] Commonly used organic solvents with a lower boiling point than acetic acid are: liquid ammonia, liquid sulfur dioxide, methylamine, dimethylamine, petroleum ether, ethyl ether, pentane, methylene chloride, carbon disulfide, solvent naphtha, acetone, 1,1-dichloro Ethane, chloroform, methanol, tetrahydrofuran, hexane, trifluoroacetic acid, 1,1,1-trichloroethane, carbon tetrachloride, ethyl acetate, ethanol, butanone, benzene, cyclohexane, acetonitrile, Isopropanol, ethylene glycol dimethyl ether, trichloroethylene, triethylamine, propionitrile, heptane, water, nitromethane, 1,4-dioxane, toluene, nitroethane, pyridine, 4 -Methyl-2-pentanone.
[0040] Combining the physical and chemical properties of goserelin and PLGA, especially their solubility in the above-mentioned solvents, the above-mentioned solvents were initially screened.
[0041] During the test, it was found that, except for water, ethanol, ether, a...
Embodiment 2
[0053] Example 2: Comparison of the effect of mixed solvent and single solvent acetic acid
[0054] In view of the fact that the solvent used in the preparation process of goserelin implants also has a significant impact on the release, related substances, content uniformity, content and residual solvent of the product, the mixed solvent and acetic acid obtained by preliminary screening in Example 1 The experiment is compared.
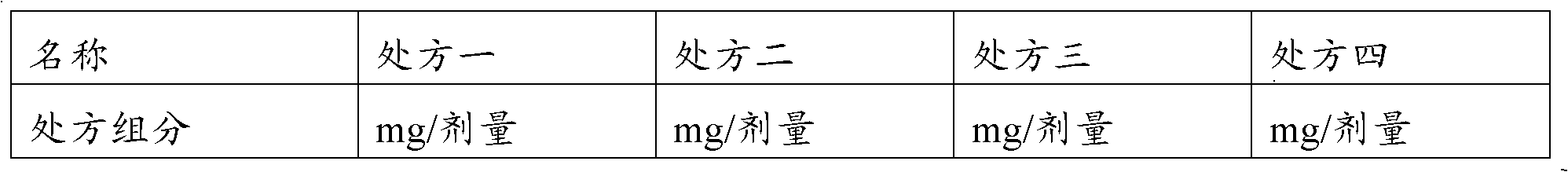
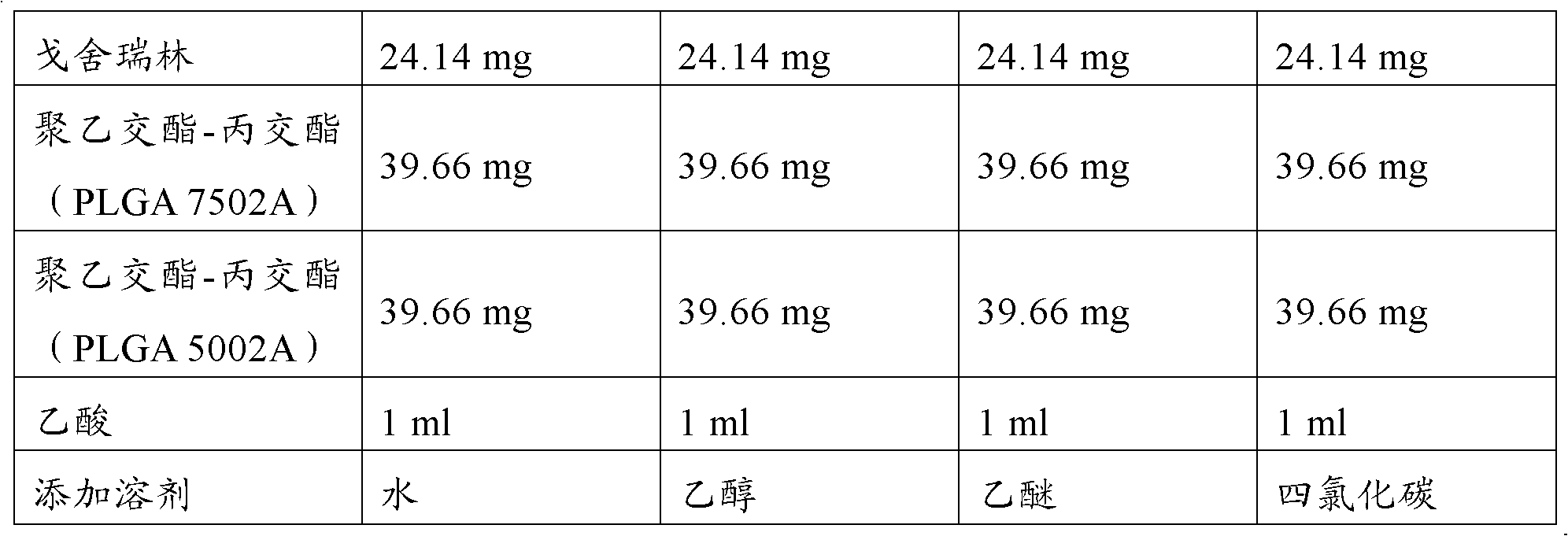
[0055] Choose a mixed solvent (acetic acid and ethanol) and a single solvent acetic acid to test. The experimental design is shown in Table 3.
[0056] Table 3 Comparison test prescription of mixed solvent and single solvent acetic acid
[0057]
[0058] Preparation Process:
[0059] 1. Dissolve. Weigh the prescription amount of goserelin, PLGA 5002A and PLGA 7502A powder or granules, add an appropriate amount of mixed solvent according to the prescription amount, and dissolve it completely into a solution under the action of stirring;
[0060] 2. Dry. Dryin...
Embodiment 3
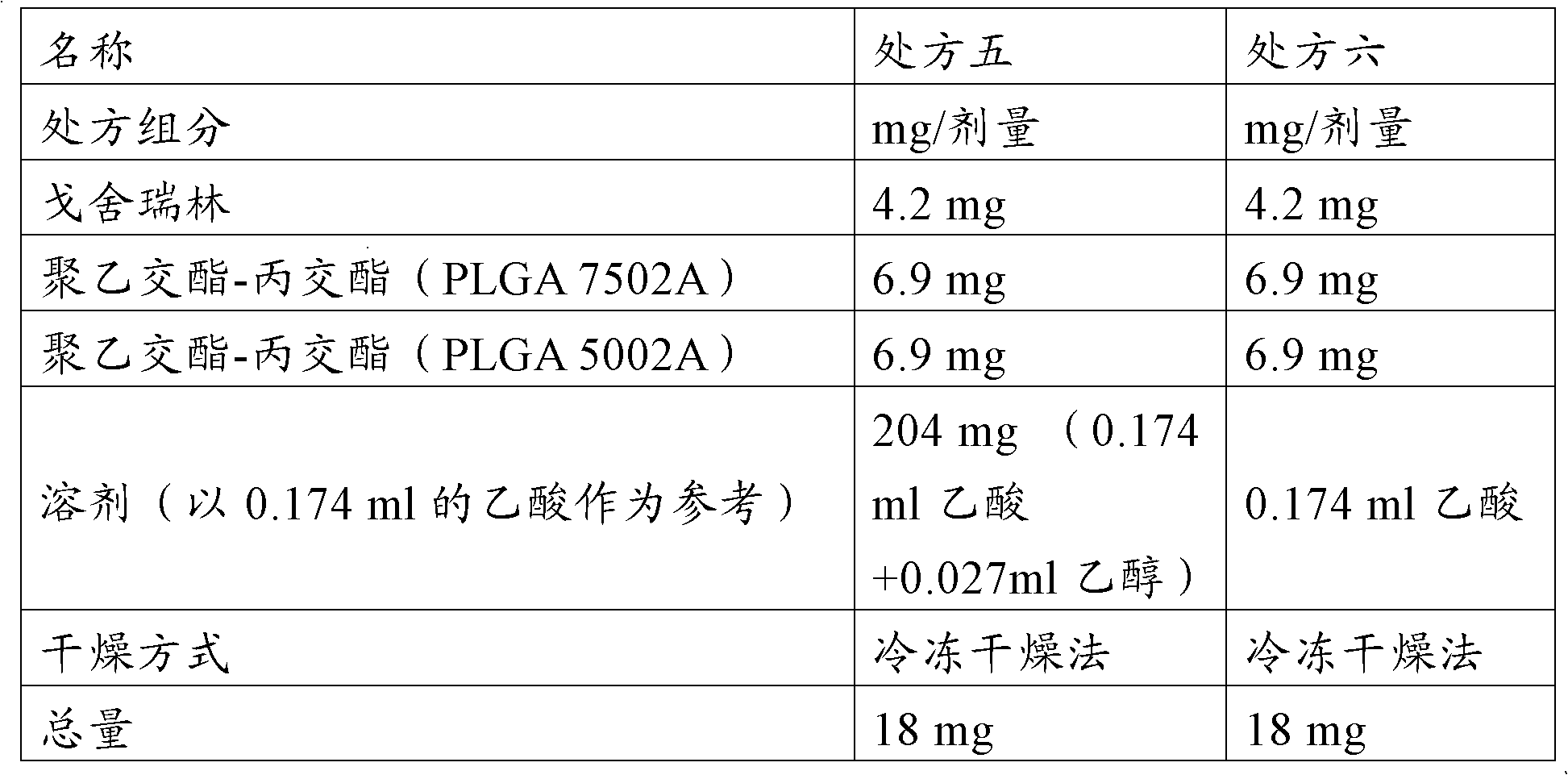
[0225] Example 3: Using mixed solvents (acetic acid and ether) to prepare goserelin sustained-release implants
[0226] prescription:
[0227]
[0228] Preparation Process:
[0229] 1. Dissolve. Weigh the prescription amount of goserelin, PLGA 5002A and PLGA 7502A powder or granules, add an appropriate amount of mixed solvent according to the prescription amount, and dissolve it completely into a solution under the action of stirring;
[0230] 2. Dry. Drying is carried out according to the drying method in the formulation design. The process parameters for the solution to remove the solvent by freeze-drying are: pre-freeze at -20°C for 4 hours, and then at 1°C / hour, the descending rate is reduced to -50°C, and stored for 20 hours Afterwards, the temperature is raised to room temperature at 20°C / hour to prepare porous spongy fine powder;
[0231] 3. Forming. Place the collected particles or fine powder in a hot melt extruder, and extrude them under the conditions of a melting temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com