Method for electrolyzing metals through wet method
A technology for electrolysis of metals and wet processes, which is applied in the improvement of process efficiency, photographic technology, instruments, etc., can solve the problems of electrolysis voltage drop, electrolyte purification and impurity removal, and energy consumption reduction, so as to improve the leaching rate and improve the utilization rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
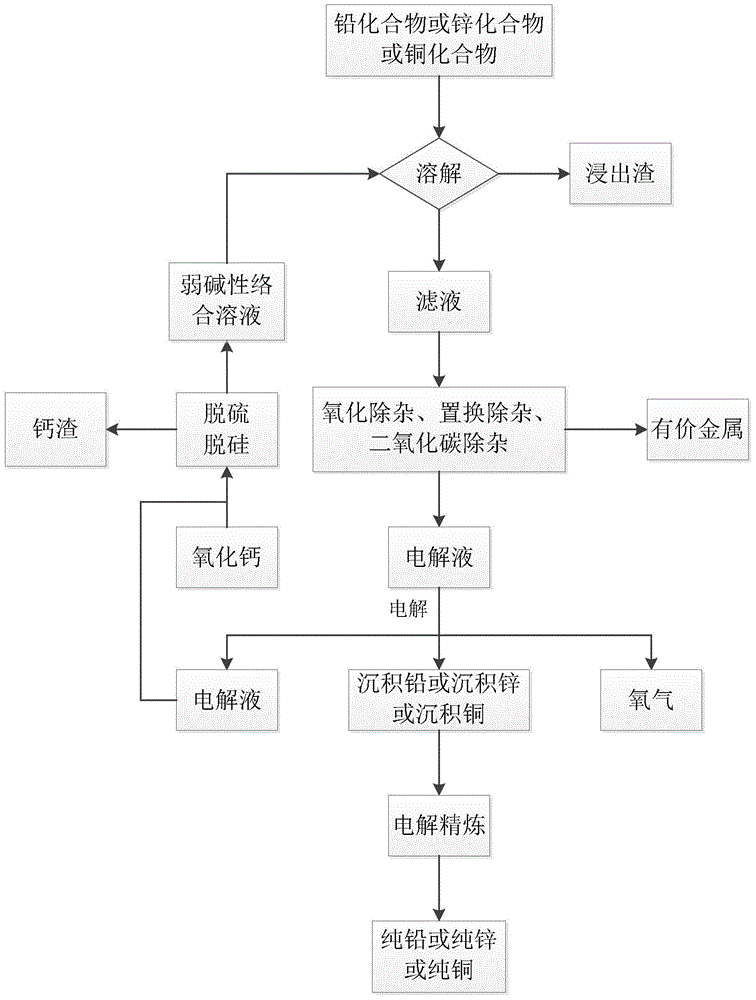
[0086] 1) Configure 10L of weakly alkaline complexing solution containing 1mol / L ethylenediamine, 0.7mol / L phenylalanine, 0.1mol / L zinc oxide, 1g / L gelatin, 2.5mol / L sodium sulfate, and adjust with sulfuric acid The pH of the solution is between 9.5-10.0, 1.2 kg of zinc sulfide calcine containing 4% lead, 3% copper, and 60% zinc is added, and reacted at 70°C for 0.5 hours.
[0087] 2) The solution obtained in step 1) is filtered, and excess zinc powder is added to the filtrate to remove lead and copper ions contained in the solution.
[0088] 3) Filter the solution obtained in step 2), and pass oxygen into the filtrate to convert the ferrous ions in the solution into iron hydroxide precipitates.
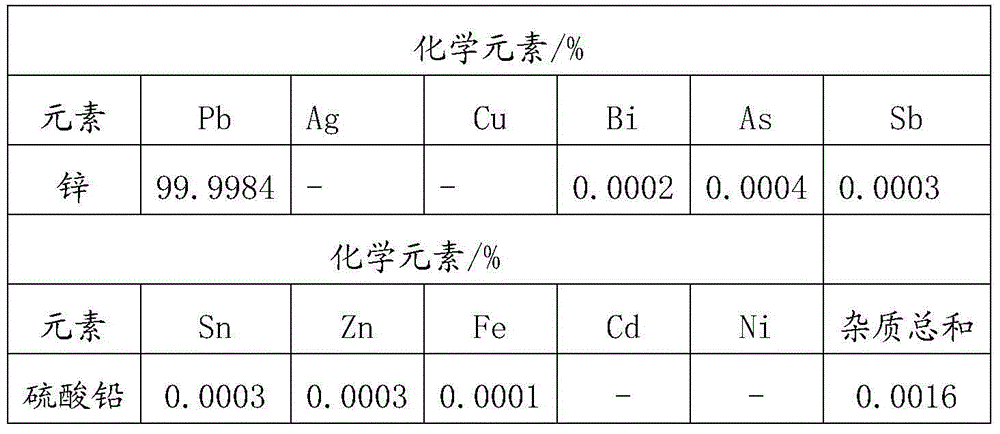
[0089] 4) Filter the solution obtained in step 3) and inject it into the electrolytic cell. The anode uses graphite electrode with an area of 500cm 2 , The cathode adopts pure aluminum inert electrode, with an area of 500cm 2 , Constant current electrolysis at a constant temperature of ...
Embodiment 2
[0092] 1) Take 10kg of lead paste from used lead-acid batteries and thermally decompose it at 550°C for 30 minutes to decompose all lead dioxide into lead oxide.
[0093] 2) Configure 25L weakly alkaline complexing solution containing 0.5mol / L phenylalanine, 0.4mol / L arginine, 0.1mol / L lead oxide, 0.1g / L rosin, 0.5mol / L potassium sulfate, using Adjust the pH value of the reaction solution with potassium hydroxide aqueous solution to be between 8.2 and 8.5, add 3.5 kg of the heat-treated lead paste in step 1), and react at 60° C. for 1.5 hours.
[0094] 2) The solution obtained in step 1) is filtered, and excess metallic lead is added to it to remove trace copper contained therein.
[0095] 3) Filter the solution obtained in step 2) and inject it into the electrolytic cell. The anode uses 5 titanium electrodes with an area of 500 cm 2 , The cathode adopts 6 pieces of pure lead chip electrodes with an area of 500cm 2 , Constant temperature 75℃, current density 450A / m 2 Under consta...
Embodiment 3
[0100] 1) Take 1 kg of copper ore containing 50% of basic copper carbonate and thermally decompose it at 400° C. for 1 hour to decompose basic copper carbonate into carbon dioxide and copper oxide.
[0101] 2) Configure 5L weakly alkaline complexing solution containing 1.3mol / L histidine, 0.5mol / L copper oxide, 0.1g / L polyethylene glycol, 1.8mol / L sodium chloride, and adjust with potassium hydroxide aqueous solution The pH value of the reaction solution is between 10.5-11.0, 300 g of the copper oxide ore obtained in step 1) is added, and the reaction is carried out at 90° C. for 0.5 hours.
[0102] 3) The solution obtained in step 2) is filtered, and excess metallic copper is added to it to remove trace amounts of silver contained therein.
[0103] 4) Filter the solution of step 3) and add calcium hydroxide to it to convert a small amount of silicate, sulfate and undecomposed carbonate dissolved in the ore into calcium sulfate, calcium silicate, and calcium carbonate precipitation.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com